Recommended immunization schedule for children and adolescents: Committee on Infectious Diseases of the Korean Pediatric Society, 2018
Article information
Abstract
The Committee on Infectious Diseases of the Korean Pediatric Society recommended immunization schedule for children and adolescents aged 18 years or younger in the 9th (2018) edition of Immunization guideline. This report provides the revised recommendations made by the committee and summarizes several changes from the 2015 guideline. National immunization program (NIP) launched a human papillomavirus (HPV) immunization for girls aged 12 years in 2016. NIP has also expanded age indication for inactivated influenza vaccine (IIV) to 12 years of age in the 2018-2019 season. Quadrivalent IIVs with a full dose (0.5 mL) are approved for all children of 6 months or older. Recommendations of live attenuated influenza vaccine were removed. For inactivated Japanese encephalitis vaccine, first 2 doses are considered as the primary series. Recommendations for use of newly introduced vaccines (diphtheria-tetanus-acellular pertussis/inactivated poliovirus/Haemophilus influenzae type b, 9-valent HPV, new varicella vaccine, new quadrivalent IIV, and attenuated oral typhoid vaccine) were added. Lastly, monitoring system for adverse events following immunization was updated. Other changes can be found in the 9th edition of Immunization guideline in detail.
Introduction
The recommended immunization schedule for children and adolescents of 18 years or younger in Korea was revised on October 2018. The Committee on Infectious Diseases of the Korean Pediatric Society (KPS) released the 9th edition of Immunization guideline [1], updated version of the previous 8th edition [2] published on 2015. This report focuses on the introduction of the revised recommendations on the childhood immunization schedule to pediatricians and other immunization providers. In addition, this report summarizes several changes in immunization practice in the Republic of Korea between 2015 and 2018.
National immunization program (NIP) introduced human papillomavirus (HPV) immunization for girls aged 12 years in 2016. A 2-dose series of either 2-valent or 4-valent HPV vaccine is recommended at six-month interval. NIP had also launched trivalent inactivated influenza vaccine (IIV) for infants and children of 6 to 59 months of age in the 2017-2018 season and expanded age indication to 12 years in the 2018–2019 season. Although children under 5 years of age are at high risk for serious complications of influenza, the committee agreed on the main purpose of age expansion to prevent the spread of influenza from school-aged children to the community. Other changes in influenza vaccine are as follows: quadrivalent IIV of full dose (0.5 mL) may be used for all children of 6 months or older. Recommendations of live attenuated vaccine were removed because it is no longer available for use in Korea. For inactivated Japanese encephalitis vaccine, first 2 doses are considered as the primary series and third dose is a booster dose. Recommendations for the use of newly introduced vaccines were added. Diphtheria-tetanus-acellular pertussis/inactivated poliovirus/Haemophilus influenzae type b vaccine (Pentaxim, Sanofi Pasteur SA., Lyon, France), 9-valent HPV vaccine (Gardasil 9, Merck & Co., Inc., Whitehouse Station, NJ, USA), new varicella vaccine (SKY Varicella, SK Bioscience, Andong, Korea), inactivated quadrivalent influenza vaccines (GC FLU Quadrivalent, GC Pharma, Yongin, Korea; SKY Cellflu Quadrivalent, SK Bioscience; FluarixTetra, GlaxoSmithKline, Middlesex, UK; and Vaxigrip Tetra, Sanofi-Pasteur SA) were introduced. Lastly, monitoring system for adverse events following immunization (AEFI) was updated. Along with a surveillance system for AEFI that has been maintained for NIP vaccines by Korea Centers for Disease Control and Prevention (KCDC), Korea Institute of Drug Safety (KIDS) collects AEFI as well. By sharing the data between KCDC and KIDS, a better comprehensive safety surveillance system is under construction. More details on recommendations and changes can be found in the 9th edition of Immunization guideline.
Recommended immunization schedule
Table 1 shows the recommended immunization schedule for children and adolescents aged 0 through 18 years. These recommendations must be read with the following notes at the bottom of the table. As previously mentioned, age expansion of IIV to 12 years and a 2-dose series of HPV vaccine are the main changes in the current recommended schedule. It is important to note that the committee decided not to recommend the meningococcal quadrivalent conjugate vaccine for healthy children who are not at increased risk of meningococcal disease, based on the epidemiology of invasive meningococcal diseases in Korea [3]. Pediatricians and other immunization providers should always ensure that they are following the most up-to-date schedules, which are available from each chapter of the 9th Immunization guideline 2018, newsletters including the important updates on the recommendations, and the KPS website at http://www.pediatrics.or.kr.
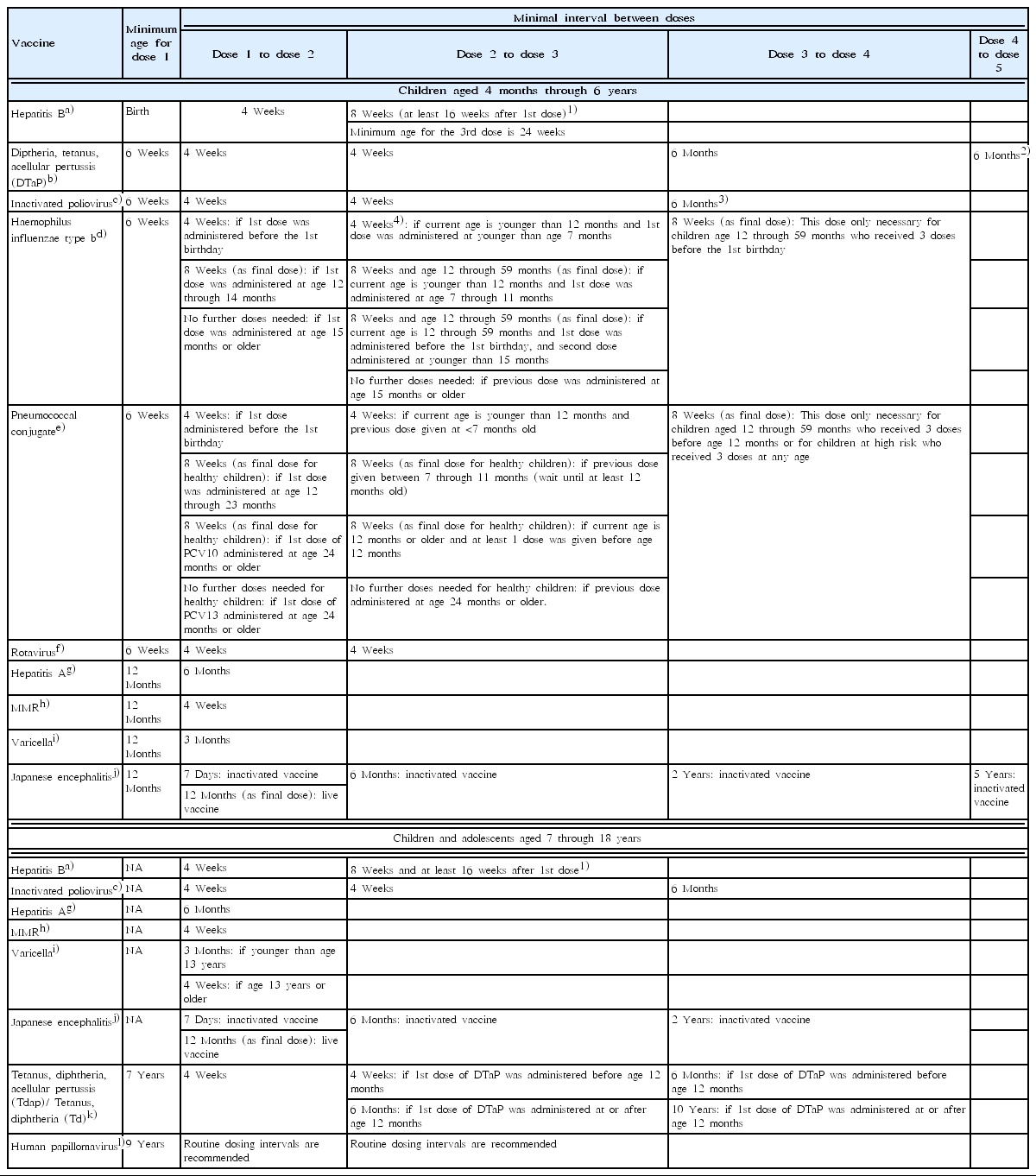
Catch-up immunization schedule
For those who start vaccine late or are behind the schedule, catch-up immunization schedule is indicated in Table 2. For children and adolescents whose vaccination has been delayed for more than 1 month, the catch-up immunization schedule should be referred to. The catch-up schedule consists of 2 parts; one for infants and preschoolers aged 0 through 6 years, the other for children and adolescents from 7 to 18 years of age.
Conclusion
The current KPS recommendation of immunization proposes that all children and adolescents residing in or planning to reside in Korea should be immunized according to the schedules provided in this report. Pediatricians and other vaccine providers should be aware of these updated recommendations and advice parents and caregivers on proper immunization practices based on these updated guidelines.
Notes
No potential conflict of interest relevant to this article was reported.