Treatment of refractory IgA vasculitis with dapsone: a systematic review
Article information
Abstract
IgA vasculitis, formerly known as Henoch-Schönlein purpura, is a systemic IgA-mediated vasculitis of the small vessels commonly seen in children. The natural history of IgA vasculitis is generally self-limiting; however, one-third of patients experience symptom recurrence and a refractory course. This systematic review examined the use of dapsone in refractory IgA vasculitis cases. A literature search of PubMed databases retrieved 13 articles published until June 14, 2018. The most common clinical feature was a palpable rash (100% of patients), followed by joint pain (69.2%). Treatment response within 1–2 days was observed in 6 of 26 patients (23.1%) versus within 3–7 days in 17 patients (65.4%). Relapse after treatment discontinuation was reported in 17 patients (65.4%) but not in 3 patients (11.5 %). Four of the 26 patients (15.4%) reported adverse effects of dapsone including arthralgia (7.7%), rash (7.7%), and dapsone hypersensitivity syndrome (3.8%). Our findings suggest that dapsone may affect refractory IgA vasculitis. Multicenter randomized placebo-controlled trials are necessary to determine the standard dosage of dapsone at initial or tapering of treatment in IgA vasculitis patients and evaluate whether dapsone has a significant benefit versus steroids or other medications.
Key message
Question: Is dapsone effective in reftractory IgA vasculitis?
Finding: Treatment response within 7 days was observed in 88.5% patients. Treatment discontinuation without tapering seems related to relapse because 15 patients reported prompt recurrence, whereas the tapering group reported fewer relapses than without tapering.
Meaning: Multicenter randomized placebo-controlled trials are necessary to determine the standard dosage of dapsone at initial or tapering of treatment in IgA vasculitis with cautions of side effects.
Introduction
IgA vasculitis, formerly known as Henoch-Schönlein purpura, is a systemic IgA-mediated vasculitis of the small vessels that is common in children. The major clinical features of IgA vasculitis are palpable purpura, arthritis, abdominal pain, and nephritis [1]. The natural history of IgA vasculitis is generally self-limiting; however, one-third of patients experience symptom recurrence and a refractory course [2]. Patients with renal disease are more likely to experience recurrence, and the long-term prognosis of IgA vasculitis is related to nephritis [1-5].
However, the treatment for IgA vasculitis has not been established. Steroids can manage abdominal pain symptoms but are unsuitable for the prevention of nephropathy [2]. Joint pain is managed with nonsteroidal anti-inflammatory drugs and steroids in severe cases [2]. Cutaneous symptoms respond to steroids and dapsone [2]. Some studies reported that immunosuppressive therapy could be considered for IgA vasculitis with nephropathy; however, the treatment of nephritis remains controversial [2]. Treatment with steroids and immunosuppressive therapy such as cyclophosphamide, cyclosporine, and azathioprine is beneficial for IgA vasculitis nephritis [1,2,6]. Some reports showed that colchicine and dapsone may be useful for treating chronic IgA vasculitis [6].
Among the diverse armamentarium of therapeutic options, dapsone has been used in several severe cases. Although it is not considered a standard treatment for IgA vasculitis, purpuric rash, abdominal pain, and arthritis in IgA vasculitis respond to it [7]. Dapsone, known as an antileprosy drug, has antioxidant scavenger effects and may suppress the production of toxic free radicals by neutrophils. It also inhibits prostaglandin D2 production and the synthesis of IgG and IgA antibodies [8]. It also inhibits IgA-neutrophil interactions, which may explain the potential efficacy of dapsone in the management of IgA vasculitis [7,9]. Therefore, in the absence of clinical trials, this systematic review aimed to elucidate the characteristics of dapsone use in refractory IgA vasculitis.
Methods
1. Search strategy
We searched PubMed for articles of IgA vasculitis treated with dapsone published until June 14, 2018. The search terms were: “Purpura” AND “Dapsone”. Two of the reviewers (SHH and YHJ) screened the articles by reading the titles, abstracts, and full texts.
The exclusion criteria were as follows:
(1) studies not related to IgA vasculitis or dapsone; (2) studies not written in English; (3) review articles; (4) animal studies; and (5) studies that are considered too old to be reliable.
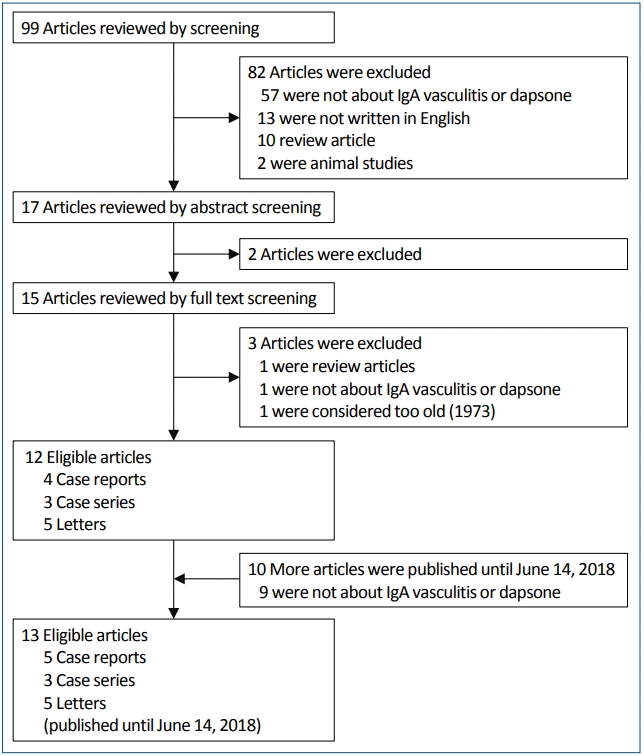
One reviewer (SHH) executed the initial search, which retrieved 99 articles published until June 24, 2015. Identification of the relevant literature was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement [10]. We excluded 86 articles after reading the abstracts and full texts according to the exclusion criteria stated above: 60 not about IgA vasculitis or dapsone, 13 not written in English, 11 were review articles, and 2 were animal studies. Of the remaining 13 articles, 1 that was published in 1973 was excluded because we considered it too old to be reliable. After about 3 years, another reviewer (YHJ) conducted the following search that retrieved 10 more articles published until June 14, 2018. Of those 10 articles, 9 not related to IgA vasculitis or dapsone were excluded. Finally, 13 articles eligible for our systematic review were identified (5 case reports, 3 case series, and 5 letters). All these studies were defined “refractory” IgA vasculitis and “positive response” to dapsone. The detailed process of article selection is presented in Fig. 1. The detailed article selection process is presented in Fig. 1.
2. Data extraction
From the 13 articles, 2 reviewers (SHH and YHJ) extracted the data and organized the information including author names, journal title, patient age and sex, dosage, clinical features, treatment response, treatment duration, postdischarge relapse, and adverse effects. When discrepancies arose, 2 reviewers (JIS and KHL) discussed them until a consensus was reached.
3. Data analysis
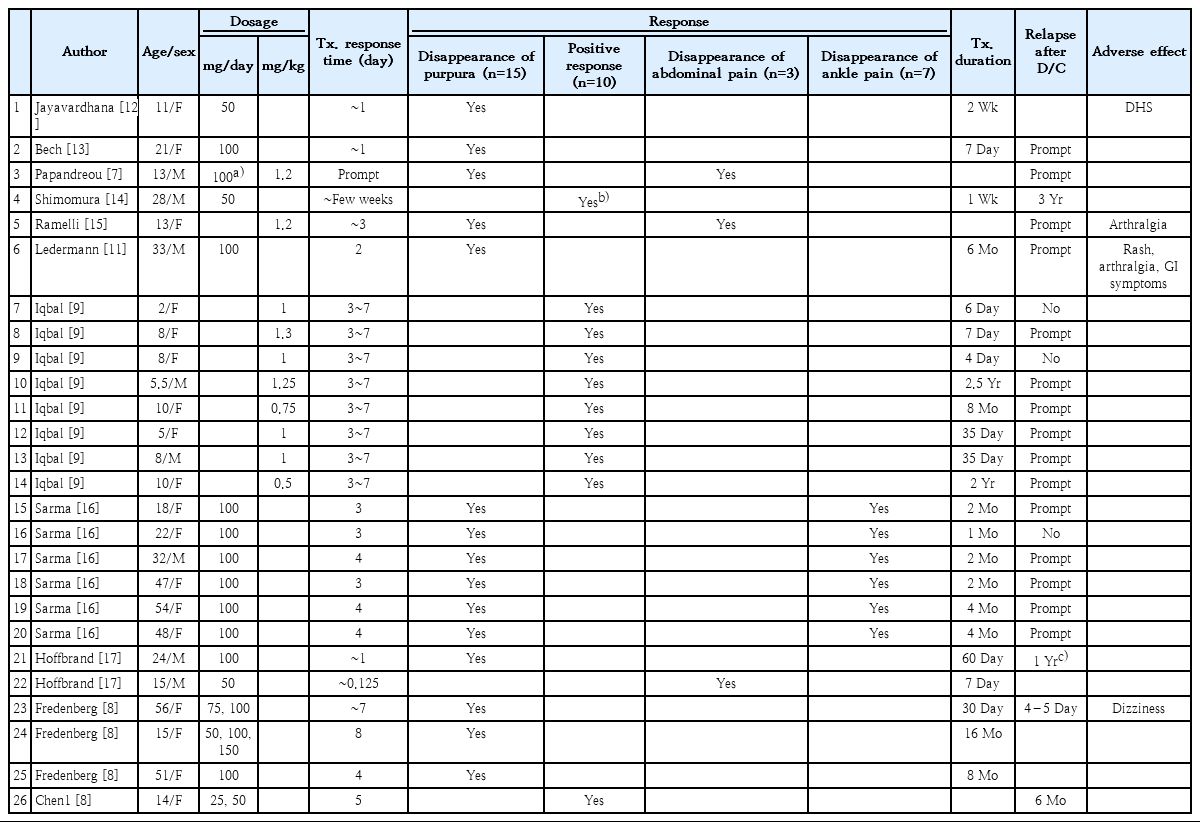
The data extracted from the articles are presented in Table 1 [7-9,11-18]. We organized the information including the patient age and sex, clinical presentation, dosage, treatment response time, treatment response, treatment duration, postdischarge relapse, and adverse effects for analyses (Tables 2, 3) [7-9,11-18].
Results
1. Presenting features of IgA vasculitis patients
Data of 26 patients with IgA vasculitis were selected from 11 articles; their clinical features are described in Table 1. The mean patient age was 21.98 years; 14 cases were pediatric. The malefemale ratio was 9:4 (18:8).
The most common clinical feature, a palpable rash, was seen in all 26 patients. Joint pain was the second most common feature (18 of 26 patients; 69.2%). Other commonly reported features were abdominal pain in 16 patients (61.5%) and hematuria in 10 patients (38.5%). Joint swelling was reported in 8 patients (30.8 %); 6 patients (23.1%) also had joint pain. Two patients (8.0%) without joint pain were female and younger than 15 years of age. Seven patients (26.9%) had vomiting. Proteinuria was identified in 6 patients (23.1%); all but 1 patient also had hematuria. Patients with proteinuria were all younger than 15 years old. Five patients had diarrhea, while all had joint pain. Every patient with vomiting and diarrhea complained of abdominal pain. Three patients (11.5%) had rectal bleeding; all were younger than 15 years of age. The bleeding was associated with hematuria and joint pain. Only 1 patient had a swollen testicle. Bullae and ulcers were simultaneously seen in 2 patients (8.0%).
Table 2 summarizes the results of 26 dapsone-treated IgA vasculitis patients by age, sex, dapsone dosage, symptom course, and adverse effects. We describe specific dapsone dosages, clinical outcomes, and adverse effects of dapsone in this section.
2. Dapsone dosage
All patients were grouped by age. Patients in group 1 were 11–56 years, while those in group 2 were 2–10 years. Age was factored into the dapsone dosing strategies; the results due to different strategies from the 2 groups are summarized in Table 4. The doses given to each patient with IgA vasculitis differed widely. Daily doses of 50–100 mg were administered in group 1, while daily doses of 0.50–1.30 mg/kg were administered in group 2.
In group 1, 11 patients took 100 mg dapsone per day and 4 patients took 50 mg per day. One patient in group 1 (not shown in Table 4) received a prescribed dapsone dose in units of milligrams per kg. Another patient in group 1 started on dapsone 75 mg every day, but it was increased to 100 mg after 3 days. In group 2, 4 of 8 patients took dapsone 1 mg/kg, and the other 4 patients were administered different doses of 0.5, 0.75, 1.25, and 1.30 mg/kg.
3. Symptom course
Symptom resolution was reported in all cases. Table 3 shows the patients’ treatment responses time, extent of response, and time to relapse after discontinuation. Treatment response within 1–2 days was observed in 6 of the 26 patients (23.1%). Within 3–7 days, 17 patients (65.4%) responded to treatment. Three patients (11.5%) showed a response after the first week.
Symptom improvements were categorized as follows: disappearance of purpura, positive response to dapsone, disappearance of abdominal pain, and disappearance of ankle pain. Of the 26 patients, 14 (57.7%) showed disappearance of purpura, 10 patients (38.5%) showed a positive response, 3 patients (11.5%) reported disappearance of abdominal pain, and 7 patients (26.9 %) reported disappearance of ankle pain.
Relapse after treatment discontinuation was reported in 17 patients (65.4%), whereas 3 patients (11.5%) reported no re lapse of symptoms. Six patients’ follow-up data regarding symp tom relapse could not be obtained. Of the 17 patients who showed symptom relapse, immediate relapse after therapy discontinuation was reported in 10 patients (58.8%), relapse within 1 year was reported in 4 patients (23.5%), and relapse beyond 1 year after discontinuation was reported in 3 patients (17.6%).
In the immediate relapse group, all but 1 patient showed immediate symptom recurrence after discontinuation and 1 patient reported relapse of symptoms 4–5 days after disconti nuation. Of the 4 patients who experienced relapse during the first year, 1 reported occasional relapse during the first 10 months, while the other 3 patients showed no recurrence within 6, 9, and 12 months after discontinuation. Of the 3 patients who showed relapse after 1 year, 1 patient reported purpura and mild arthralgia after 3 years. The other 2 patients had disease-free periods of 1.5 and 6 years (Table 3).
4. Adverse effects of dapsone
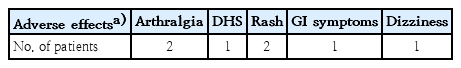
Four of the 26 patients (15.4%) reported adverse effects of dapsone. The adverse effects are summarized in Table 5. The adverse effects of dapsone for patients with IgA vasculitis included arthralgia (7.7%), dapsone hypersensitivity syndrome (3.8%), rash (7.7%), gastrointestinal symptoms (3.8%), and dizziness (3.8%). Only 1 patient reported multiple adverse effects including arthralgia, rash, and gastrointestinal symptoms.
Discussion
IgA vasculitis is a systemic IgA-mediated vasculitis characterized by palpable cutaneous purpura, arthritis, abdominal pain, gastrointestinal bleeding, and nephritis [1]. Although IgA vasculitis is usually a self-limiting disease, one report showed that 33% of patients experience a relapse [19]. The aim of drug therapy in IgA vasculitis is rapid symptomatic relief because the symptoms of IgA vasculitis are generally self-limiting with the exception of renal disease, which might become a long-term complication [1]. Steroids are considered the mainstay of treatment because they seem to have a relief effect on abdominal [20,21] and joint pain [21]. In one study of 417 patients, corticosteroids and nonsteroidal antiinflammatory drugs were commonly prescribed [22]. One report showed that azathioprine can be used to treat recurrent IgA vasculitis [23]. Factor XIII may have diagnostic value in patients with severe gastrointestinal symptoms without cutaneous rash; the infusion of activated factor XIII can be an adjunctive therapy in such patients [24]. Similarly, factor XIII and intravenous immunoglobulin therapy can be effective in IgA vasculitis patients with severe abdominal symptoms [25,26].
Despite various attempts, a standardized treatment for recurrent IgA vasculitis is lacking. Corticosteroids have various side effects; corticosteroid-resistant patients with IgA vasculitis have been treated effectively with mizoribine or cyclosporine A [27,28]. Drugs such as azathioprine, factor XIII, and intravenous immunoglobulin are expensive or exhibit potential severe side effects. In contrast, dapsone is safe and available in pill form. However, only a few cases of recurrent IgA vasculitis were treated with dapsone.
The use of dapsone in IgA vasculitis appears rela tively unfamiliar to clinicians compared to that of steroids, although the first case of IgA vasculitis treated with dapsone was reported in 1983 [29]. Dapsone is an antileprosy drug that can be used for dermatological diseases associated with the deposition of antibodies and accumulation of neutrophils [30]. Dapsone is an alter native, especially in relapsed IgA vasculitis, considering that IgA vasculitis is an IgA-mediated vasculitis and that the IgA-neutrophil interaction could be inhibited by dapsone [7,9].
The World Health Organization (WHO) regimens are the standard treatment for leprosy. The WHO recommends using dapsone 100 mg daily for 6 months in cases of paucibacillary leprosy and 100 mg daily for at least 2 years and to smear negativity in cases of multibacillary leprosy [30]. One study showed that peak serum concentrations of dapsone of 1.10–2.33 mg/L were reached within 0.5–4 hours after 100 mg was orally administered to 25 healthy volunteers [31]. However, in severe leprosy patients, absorption could be decreased [32]. In leprosy, the therapeutic range of dapsone is 0.5–5 mg/L; the adverse effect of dapsone is rare at plasma levels below 5 mg/L [33]. The most common adverse effect is methemoglobinemia, which is usually not a serious problem except at doses exceeding 200 mg/day [34]. Hemolysis and frank anemia is another well-known side effect [35]. Increasing age and the daily dose of dapsone were related to increased magnitude of hemolysis in dapsone therapy [35]. Thus, it is recommended not to exceed 1.5 mg/kg body weight or 100 mg in normal healthy persons [36]. Agranulocytosis is a fatal adverse effect of dapsone [37]. Another rare side effect is a hypersensitivity syndrome, called dapsone syndrome, which is characterized by fever, rash, and internal organ involvement [38,39]. Other side effects include neurotoxicity such as peripheral neuropathy (rare, typically at doses exceeding 300 mg/day) and psychosis (rare, at doses below 100 mg/day), which is dose-independent [40-43].
In our reviewed patients, no treatment exceeded 100 mg/ day or 1.5 mg/kg. One patient who stopped dapsone because of dizziness at 100 mg/day did not report dizziness at 150 mg/day after IgA vasculitis recurrence. This means that the side effect of dapsone is somewhat unpredictable and dose-independent. However, although many side effects of dapsone may be doseindependent, to minimize the adverse effect, the dose should not exceed 100 mg/day or 1.5 mg/kg. In our reviewed patients, treatment discontinuation without tapering seems related to relapse because 15 patients reported prompt recurrence, whereas the tapering group reported fewer relapses.
This study has the limitation that only 26 patients were reviewed. Because of the small number of patients, it is difficult to explain the use of dapsone in detail such as how to taper its use. Of our reviewed patients, 19.0% did not require dose tapering; however, because of the small number of patients, our results cannot confirm what percentage of people will need it or why.
To best of our knowledge, this is the first systematic review to summarize the efficacy of dapsone in IgA vasculitis. Since several side effects should be noted, most patients with recurrent or refractory disease should be counseled about the side effects by clini cians and start on dapsone according to the risks and benefits. However, our study will increase awareness that dapsone is an effective treatment for recurrent IgA vasculitis pati ents. Multicenter randomized placebo-controlled clinical trials are necessary to determine the standard dapsone use or dosage as a treatment option at the time of treatment initiation or tapering in refractory IgA vasculitis patients and evaluate whether it has a significant benefit compared to steroids or other medications.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was performed by medical students at Yonsei University College of Medicine. We express our most sincere gratitude to the students who assisted with the manuscript preparation.