Factors associated with seizure and cognitive outcomes after epilepsy surgery for low-grade epilepsy-associated neuroepithelial tumors in children
Article information
Abstract
Low-grade epilepsy-associated neuroepithelial tumors (LEATs) are responsible for drug-resistant chronic focal epilepsy, and are the second-most common reason for epilepsy surgery in children. LEATs are extremely responsive to surgical treatment, and therefore epilepsy surgery should be considered as a treatment option for LEATs. However, the optimal time for surgery remains controversial, and surgeries are often delayed. In this review, we reviewed published article on the factors associated with seizure and cognitive outcomes after epilepsy surgery for LEATs in children to help clinicians in their decision whether to pursue epilepsy surgery for LEATs. The achievement of gross total resection may be the most important prognostic factor for seizure freedom. A shorter duration of epilepsy, a younger age at surgery, and extended resection of temporal lobe tumors have also been suggested as favorable prognostic factors in terms of seizure control. Poor cognitive function in children with LEATs is associated with a longer duration of epilepsy and a younger age at seizure onset.
Introduction
Low-grade epilepsy-associated neuroepithelial tumors (LEATs) are responsible for drug-resistant chronic focal epilepsy that presents with seizure onset during childhood or young adulthood [1]. LEATs comprise low-grade glial or glioneuronal tumors (GNTs) such as gangliogliomas (GGs), dysembryoplastic neuroepithelial tumors (DNTs), angiocentric astrocytomas, and isomorphic astrocytomas.
After leukemias, brain tumors are the most common tumors (20%–22%) in children [2]. Brain tumors are graded based on their histological appearance and molecular parameters. World Health Organization grades 1 and 2 are benign tumors, while grades 3 and 4 are malignant. Before biopsy or surgical resection, due to multiple and nonspecific clinical signs, tumor grade is mostly assumed based on analysis of tumor site and appearance on multiparametric magnetic resonance imaging (MRI) [3]. For example, a hemorrhagic tumor with peripheral edema containing multiple ectatic vessels or a hypercellular tumor that is iso- or hypointense compared to the cortex on T2-weighted imaging is more suggestive of high-grade tumors [3-5]. Seizure prevalence is higher in slow-growing benign brain tumors, and some suggested explanations include: (1) longer life expectancy of patients with low-grade tumors contributing to increased seizure frequency; (2) insufficient time for cells of high-grade tumors to reorganize, vascularize, and develop mechanisms necessary for epileptogenesis; and (3) slow-growing tumor cells may possess intrinsic epileptogenic properties [6-8].
As the propensity for malignant progression is very low in LEATs, seizure control may be the main treatment target. Unfortunately, there is a high incidence of drug-resistant seizures among LEAT patients; consequently, although LEATs account for only 2%–5% of all brain tumors and are the etiology of seizures in only 1%–3% of pediatric epilepsy patients, LEATs are the second-most common reason for epilepsy surgery in children [9-12]. In a European study of brain specimens from 9,523 patients who underwent epilepsy surgery, the histopathologic etiology was identified as a tumor in 24% of all patients. LEATs comprised 82% of the tumors, and 84% of the LEATs were GGs or DNTs (Table 1).
LEATs are extremely responsive to surgical treatment, and more than 80% of patients achieve seizure freedom after surgery [13-16]. Therefore, epilepsy surgery should be considered a treatment option for LEATs. However, the optimal surgical timing remains controversial, and surgeries are often delayed (Table 1), mostly due to late referral after lengthy trials of antiepileptic drugs (AEDs), with a mean duration from seizure onset to surgery (i.e., duration of epilepsy) of more than 10 years [10].
To help clinicians decide whether to pursue epilepsy surgery for LEATs, here we performed a systematic literature review of the factors associated with seizure and cognitive outcomes after epilepsy surgery for LEATs in children. Articles were extracted in July 2019 using PubMed searches of titles and abstracts with the following query terms: seizure or epilepsy; low-grade epilepsy-associated neuroepithelial tumor, long-term epilepsy-associated tumor, LEAT, ganglioglioma, dysembryoplastic neuroepithelial tumor, glioma, astrocytoma, xanthoastrocytoma, or tumor; surgery or resection; and child or pediatric. The inclusion criteria for the studies were: (1) epilepsy surgery in children with histopathologically confirmed LEATs, (2) seizure or epilepsy as the main symptom and reason for the surgery, and (3) mention of statistically significant predictive factors for seizure or cognitive outcomes. The exclusion criteria were: (1) inclusion of adults and children in the study and the reporting of data such that the child-specific data could not be distinguished from those for adults, (2) inclusion of high-grade tumors. Of 290 extracted articles, 86 were excluded after initial screening due to irrelevancy, while an additional 186 were excluded after full article review due to ineligibility. Ultimately, 18 studies were included; of them, 16 addressed factors associated with seizure outcome, and 6 addressed factors associated with cognitive outcome. All included studies were retrospective.
Epileptogenesis of LEATs
The reason why almost all GNTs, including GGs and DNTs, cause seizures is not entirely understood, and various epileptogenic mechanisms of LEAT-associated epilepsy have been suggested [17]. The LEAT itself can be intrinsically epileptogenic due to the presence of hyperexcitable dysplastic neurons or a high neuronal density within the tumor as evidenced by various results from immunocytochemical studies, such as the high expression of glutamate receptor subtypes, downregulation of several gamma-aminobutyric acid (GABA) receptors, and deregulation of cation-chloride cotransporters [18-22].
LEATs can also invade normal tissues, altering neurotransmitter expression and inflammatory reactions [23]. For example, the deregulation of glutamate uptake and release by glutamate receptors on glial cells, which results in increased extracellular glutamate concentrations and decreased glial glutamate transporter expressions, has been observed [19,20,24,25]. LEATs have also been noted to activate the innate and adaptive immune systems [26,27]. Proinflammatory molecules increase neuronal excitability by enhancing extracellular glutamate concentrations and modifying the functions of glutamate and GABA receptors [28]. Immune system activation also causes upregulation of major histocompatibility complex class I molecules in neuronal cells and activates the mammalian target of rapamycin pathway [26,27,29].
Adjacent cortical areas can also undergo dysplastic reorganization, leading to hyperexcitability [30,31]. Studies have shown that the afferentiation of adjacent cortical regions can lead to denervation hypersensitivity, modified synchronization of local networks, and overexpression of neurotransmitters in adjacent cortical areas [7,23,32].
Other suggested mechanisms for LEAT-related seizures include blood-brain barrier dysfunction, altered gap junction channels in glial cells, alterations in the surrounding neuronal network, altered regional metabolism and pH, regional hypoxic effects on the surrounding tissue, and an altered vascular supply [7,18,33-39]. Genetic predispositions for tumor-associated seizures have also been studied [7,40]. Therefore, the etiology of tumor-induced seizures is multifactorial and extends beyond the physical size of the tumor itself [41,42].
Factors associated with seizure outcomes after epilepsy surgery for LEATs
The following factors were mentioned at least once among the 16 studies that addressed seizure outcome as being predictive of seizure recurrence after surgery: younger age at seizure onset [43]; older age at surgery [44,45]; longer duration of epilepsy [44,46]; greater number of AEDs taken at the time of surgery [47]; presence of generalized seizure [48]; presence of generalized epileptiform discharges (EDs) in an electroencephalogram [49]; extratemporal or parietal location of the tumor [45]; presence of satellite lesions on a MRI scan [50]; lesionectomy of temporal tumors [43]; and subtotal tumor resection [15,45,46,51-55]. The included studies that examined seizure outcome are summarized in Table 2, and more detailed information regarding the tumor types included, parameters for seizure outcome assessment, and factors irrelevant to seizure outcome are shown in Supplementary Table 1.
Degree of tumor resection
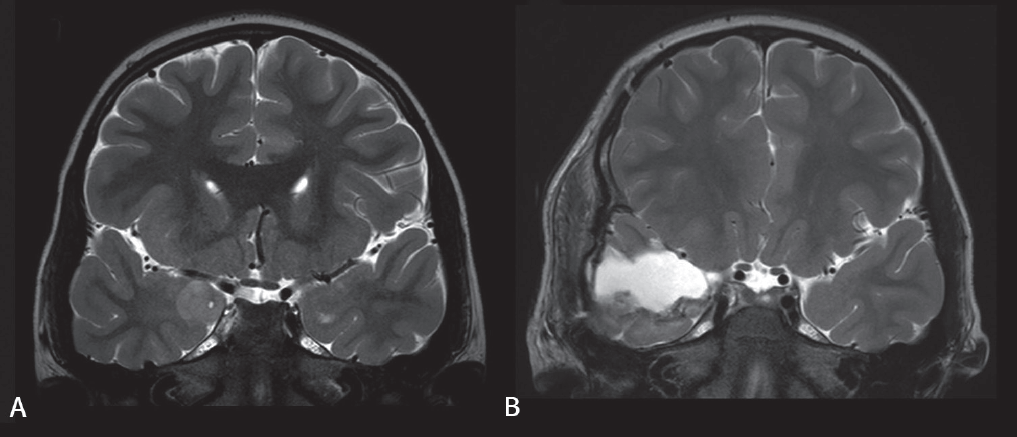
Gross total resection was the most frequently suggested favorable prognostic factor [15,45,46,49-55]. Fig. 1 shows an example of successful gross total resection of a LEAT (ganglioglioma) in a 7-year-old boy performed due to uncontrolled seizures after he took 3 AEDs for 1.3 years. Several studies that included large numbers of patients confirmed that gross total resection is among the strongest factors leading to seizure freedom after LEAT resection [41,56-58]. However, some studies have stated otherwise [49,50,59,60]. Gross total resection may not be possible if the tumor is located adjacent to eloquent areas or other functional cortical areas. Nevertheless, gross total resection should be the goal of surgery, and efforts should be made using multimodal approaches to maximize the extent of resection.
Duration of epilepsy
Two studies mentioned that a longer duration of epilepsy was a poor prognostic factor for seizure outcomes [44,45]. The same has been demonstrated in previous systematic reviews that examined adults or both adults and children [56,57,61]. Early seizures may promote progressive changes in synaptic plasticity and cerebral blood flow, with prolonged epilepsy making surrounding neurons more epileptic; thus, seizure control can become more difficult once a period of time has elapsed following seizure onset [56,62-65]. However, 8 studies found that epilepsy duration is not predictive of seizure outcome [15,43,46,49-52,55], while 2 studies suggested otherwise. Thus, more studies are needed to draw a definitive conclusion.
Age at surgery
Two studies found that an older age at surgery is predictive for persistent seizures [44,46], whereas 9 studies found no association between age at surgery and seizure outcome [15,43,45,47,48,50,52, 54,55,66]. In an Italian nationwide study of epilepsy surgery for LEATs that included a total of 282 adults and children, older age at surgery was the most significant predictor of persistent seizures, with a 4% increase in the probability of an unfavorable outcome for every year waited [16]. Since previous studies demonstrated no significant difference in surgical outcome between pediatric and adult patients with LEATs [16,56], the reason for the poor prognosis associated with older age at surgery may be the duration of epilepsy rather than the patient’s age itself.
Extent of resection
One article suggested that extended resection (i.e., removal of the tumor and surrounding epileptogenic zone) is helpful for achieving seizure freedom [43], whereas 6 other articles found no difference in seizure outcomes between lesionectomy alone and extended resection [46,47,49,52,53,66]. Thus, the matter of surgical strategy remains controversial. The discordance among previous studies also raises an important question regarding the use of additional corticectomy or additional amygdalohippocampectomy for temporal lobe tumors [14,16,23,56,60,67-69]. Extended resections are mainly performed for temporal tumors, while there is a lack of reports detailing outcomes for extratemporal tumors [43,67,69-71]. One indicator that endorses the application of extended tailored resection is the presence of adjacent dual pathology, such as focal cortical dysplasia or hippocampal sclerosis, that can cause seizures after lesionectomy [56]. Therefore, what can currently be said with confidence is that extended resection may be considered, particularly when treating temporal tumors with evidence of dual pathology [56].
Utilization of intraoperative electrocorticography
Intraoperative electrocorticography (ECoG) or 2-stage surgery is performed when delineation of the epileptogenic zone is needed due to the presence of multifocal epileptogenic foci or a tumor location adjacent to functional areas. All 3 studies that investigated the association between the utilization of intraoperative ECoG and seizure outcome found that it is not related to higher rates of seizure freedom [49,66,72]. Other large reviews reported the same conclusion. However, caution must be taken when interpreting these results, as intraoperative ECoG is more likely to be used in more difficult cases that involve multifocal epileptogenic foci or tumors located near eloquent areas [56,57]. Other studies advocate for the utilization of intraoperative ECoG during tailored surgery [73-76]. Therefore, the use of intraoperative ECoG is advised when extended resection is anticipated [73].
Factors associated with cognitive outcome after epilepsy surgery for LEATs
Studies that addressed factors associated with preoperative full-scale intelligence quotient (FSIQ), longer duration of epilepsy, and younger age at seizure onset are universally mentioned as poor prognostic factors for preoperative FSIQ [15,16,52,54,55]. An explanation of this finding is reduced brain plasticity and a limited degree of possible postoperative cognitive gains [77,78]. The studies addressing cognitive outcome are summarized in Table 3, and more detailed information regarding the cognitive parameters is shown in Supplementary Table 2.

Summary of articles addressing factors associated with cognitive outcomes after epilepsy surgery for low-grade epilepsy-associated neuroepithelial tumors
Postoperative cognitive function has been shown to significantly depend on preoperative cognitive function [15,54,55]. García-Fernández et al. [52]. reported that none of the various cognitive domains of a postoperative neuropsychological test performed 1 year after resection showed significant decline; on the contrary, there were statistically significant improvements in several cognitive domains (Supplementary Table 2). Ramantani et al. [55]. also reported that, at the group level, there was significant intraindividual improvement in verbal intelligence quotient (IQ) and performance IQ as well as a trend toward FSIQ improvement after epilepsy surgery. Finally, a study by Faramand et al. [15]. showed that postoperative FSIQ improved in 61% of children, declined in 36.5% of children, and was unchanged in 2.5% of children. The study by García-Fernández et al. [52]. mentioned above also showed poorer preoperative cognitive function in children with drug-resistant epilepsy, suggesting that it can be beneficial for surgery to occur before drug-resistant epilepsy develops.
Overall, earlier surgery can prevent low postoperative FSIQ in children, particularly young children, and that improved cognitive function can be expected following epilepsy surgery.
Conclusion
LEATs usually develop in children and young adults who present with seizures that are highly drug-resistant. Surgical treatment, however, is extremely effective, resulting in seizure freedom in approximately 70%–80% of cases. The achievement of gross total resection may be the most important prognostic factor for seizure freedom. Shorter duration of epilepsy, younger age at surgery, and extended resection of temporal lobe tumors have also been suggested as favorable prognostic factors for seizure control.
Poor cognitive function in children with LEATs is strongly associated with longer duration of epilepsy and younger age at seizure onset. Therefore, surgical treatment should be considered as an early option in children with LEATs prior to the diagnosis of drug-resistant epilepsy to protect the cognitive function of LEAT patients by averting recurrent seizures and the administration of multiple AEDs.
Supplementary materials
Supplementary Tables 1 and 2 can be found via https://doi.org/10.3345/kjp.2019.01151.
Detailed summary of literatures on factors associated with seizure outcome in epilepsy surgery of low-grade epilepsy-associated neuroepithelial tumors
Detailed summary of literatures on factors associated with cognitive outcome in epilepsy surgery of low-grade epilepsy-associated neuroepithelial tumors
Notes
No potential conflict of interest relevant to this article was reported.