Risk factors for BiPAP failure as an initial management approach in moderate to late preterm infants with respiratory distress
Article information
Key message
Question: Which factors can predict BiPAP failure as the initial management approach for moderate to late preterm infants with respiratory distress?
Finding: RDS aggravation and increased oxygen and frequency requirements during BiPAP support were associated with BiPAP failure.
Meaning: Early changes to invasive ventilator care should be considered for moderate to late preterm infants showing RDS aggravation and increased oxygen and frequency requirements during BiPAP support.
To the editor,
Noninvasive ventilation (NIV) has been increasingly used instead of intermittent mandatory ventilation (IMV) as the initial respiratory management approach in preterm infants. Among various NIV strategies, bilevel positive airway pressure (BiPAP) was introduced in recent years as an alternative to conventional nasal continuous positive airway pressure (nCPAP). Theoretically, BiPAP should enable greater alveolar recruitment, a higher residual functional capacity, and reduced breath work compared to nCPAP [1,2]. Three retrospective observational studies and 2 randomized controlled trials reported the superiority of BiPAP over nCPAP for oxygenation and ventilation in preterm infants, although there are discrepancies among the reported results [3-7]. To the best of our knowledge, no study has reported the predictive risk factors for BiPAP failure. This study aimed to investigate risk factors for BiPAP failure as the initial respiratory management strategy in moderate to late preterm infants with respiratory distress.
This retrospective observational study included patients in the neonatal intensive care unit of Korea University Ansan Hospital from January 2014 to September 2018 who required BiPAP as the initial respiratory support within 24 hours after birth. Infants who required resuscitation with intubation in the delivery room and those who were diagnosed with chromosomal abnormalities or an air leak, cardiovascular instability, or multiple congenital anomalies were excluded. Since prophylactic surfactant administration is recommended for preterm infants who are born at a gestational age (GA) of less than 30+0 weeks or with a body weight (BW) less than or equal to 1,250 g in South Korea, they were excluded from the study.
One hundred twenty-two preterm infants were categorized into the success and failure groups. The success group included preterm infants successfully weaned from BiPAP within 7 days, while those in the failure group failed weaning and required intubation under any of the following circumstances: (1) aggravation of respiratory acidosis on a blood gas analysis after BiPAP application; (2) a fraction of inspired oxygen in the air greater than 0.4 to maintain a peripheral capillary oxygen saturation of 88%–94%; and (3) more than 2–3 apnea episodes per hour requiring repeated stimulation or bag-and-mask ventilation.
Detailed data were collected, including information about pregnancy, labor, BiPAP settings, serial lab results during BiPAP, and causes of respiratory distress. Outcomes include rate of BiPAP failure; length of hospital stay; and the incidence of pneumothorax, bronchopulmonary dysplasia, symptomatic patent ductus arteriosus, periventricular leukomalacia, intraventricular hemorrhage (grade ≥II), and necrotizing enterocolitis (stage ≥2).
A significant intergroup difference was defined as a P value less than 0.05 using an appropriate statistical test for categorical data (χ2), continuous parametric data (Student t test), and nonparametric (Wilcoxon Mann-Whitney) data. Variables statistically different between the success and failure groups were included in a multivariable logistic regression model in which BiPAP failure was the dependent variable.
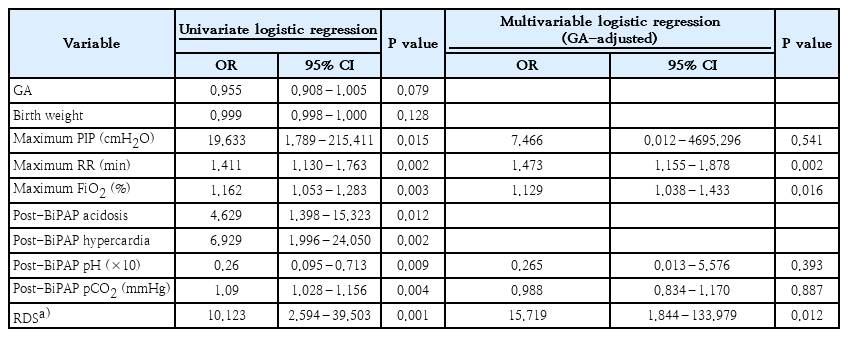
Of 122 preterm infants in this study, the rate of BiPAP failure was 10.7% (13 of 122). Most of the patients’ perinatal characteristics and maternal medical histories were similar between groups (Table 1). Nevertheless, BiPAP failure rates tended to increase as GA or BW decreased (data not shown). The incidence of respiratory distress syndrome (RDS) was significantly higher in the failure group. The need for surfactant administration was also higher in the failure group. The failure group also had a significantly higher oxygen frequency requirement during BiPAP support (Table 2). After adjustment for GA, multivariate analysis revealed that RDS, oxygen requirement, and increased frequency during BiPAP support remained significant.
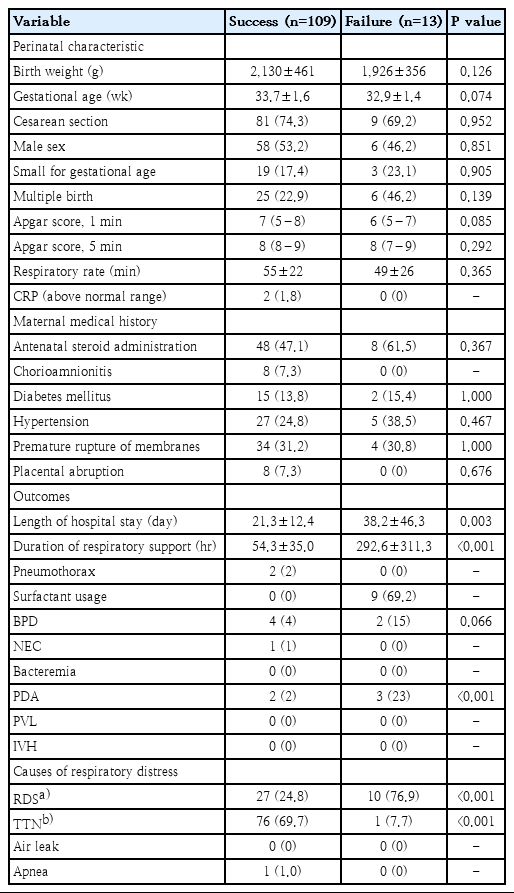
Perinatal characteristics, maternal medical history, outcomes, and causes of respiratory distress of 122 infants by study group
Regarding hospital outcomes, superiority over primarily practiced IMV was not noted in cases of NIV failure. Regarding nCPAP, Dargaville et al. [8] reported that failed nCPAP is not superior to primary intubation. Their previous study also demonstrated that RDS is the single most important antecedent to nCPAP failure in preterm infants [9]. Likewise, early prediction of BiPAP failure by checking the risk factors might enhance the efficacy of BiPAP, enabling prevention of additional morbidities in preterm infants.
Prenatal risk factors are important in the early prediction of NIV. In a study by Dargaville et al. [8], lower GA and BW, incomplete exposure to antenatal glucocorticoids, and cesarean section (C/sec) were prenatal risk factors for nCPAP failure in very and extremely preterm infants. We found no significant association between most prenatal characteristics and BiPAP failure.
In our study, RDS in moderate to late preterm infants was the single most important cause of respiratory distress in cases of BiPAP failure (Table 2). Most clinicians choose to administer surfactant in deteriorating courses of RDS. However, an evident pattern of RDS on a chest X-ray may be one of the fastest tools to predict BiPAP failure, as preterm infants in whom nCPAP fails were more likely to have chest X-ray findings consistent with severe RDS [9].
There was a data limitation regarding GA and BW due to unconditional and prophylactic surfactant administration via endotracheal tube in the delivery room. Rates of BiPAP failure in some studies differed widely (12.9%–26.6%) [3-6]. Differences in inclusion criteria among studies might have caused differences in the BiPAP failure rates among studies.
To date, BiPAP support strategies are based on clinicians’ decisions without a universal consensus. Our data showed that perinatal characteristics such as a lower GA and BW were not risk factors for BiPAP failure in infants born at a GA ≥30+0 weeks and BW >1,250 g. Since RDS was significantly correlated with BiPAP failure, early detection of RDS aggravation is important to predict BiPAP failure.
Therefore, we concluded that for moderate to late preterm infants showing RDS aggravation on BiPAP regardless of GA or BW, early changes made to invasive ventilator care or additional surfactant administration should be considered. In addition, increased oxygen and frequency requirements during BiPAP support may help identify preterm infants at high risk of BiPAP failure. Strategies to avoid NIV including BiPAP failure should be established; further multicentered well-designed randomized studies are needed to ensure the safety of BiPAP as the initial respiratory management approach for moderate to late preterm infants.
Notes
No potential conflict of interest relevant to this article was reported.