Epidemiology and clinical features of coronavirus disease 2019 in children
Article information
Abstract
Coronavirus disease-2019 (COVID-19), which started in Wuhan, China, in December 2019 and declared a worldwide pandemic on March 11, 2020, is a novel infectious disease that causes respiratory illness and death. Pediatric COVID-19 accounts for a small percentage of patients and is often milder than that in adults; however, it can progress to severe disease in some cases. Even neonates can suffer from COVID-19, and children may spread the disease in the community. This review summarizes what is currently known about COVID-19 in children and adolescents.
Introduction
In December 2019, a cluster of patients with pneumonia of unknown etiology were identified in Wuhan, China. A previously unknown betacoronavirus was detected in respiratory samples from the patients [1]. The virus was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and was deemed caused by coronavirus disease 2019 (COVID-19) [2]. COVID-19 rapidly swept mainland China and spread worldwide, causing 118,319 confirmed cases and 4,292 deaths in 113 nations as of March 11, 2020. The World Health Organization (WHO) declared COVID-19 a pandemic [3]. In the Republic of Korea, starting with a Chinese traveler from Wuhan in January 20 [4], 7,755 confirmed cases leading to 60 deaths have occurred as of March 11, 2020 [5].
COVID-19 is a novel infectious disease that is forecasted to have an enormous effect worldwide. This article reviews what is known about COVID-19 in children and adolescents as of March 12, 2020 in an effort to help clinicians managing pediatric cases.
Epidemiology in children
Published data on COVID-19 focuses primarily on adults, and the infection rate of COVID-19 in children is relatively low [6]. The first pediatric case was reported on January 20, 2020, in a 10-year-old boy from Shenzhen, China, whose family had visited Wuhan City [7]. However, a retrospective study that enrolled 366 children (≤16 years of age) hospitalized for respiratory infections between January 7 and 15, 2020 showed that COVID-19 was confirmed in 6 children (1.6%) whose onset of illness occurred between January 2 and 8, 2020. This study result suggests that SARS-CoV-2 infections in children were occurring early on in the epidemic [8].
Limited data are available on prevalence of COVID-19 in pediatric populations because children were rarely tested for the virus in earlier phase of the outbreak, especially in Hubei Province in China, where the most cases were confirmed [9]. Until January 31, 2020, of 11,791 confirmed COVID-19 cases in mainland China, 74 cases of pediatric patients (0.6%) aged 1.5 months to 18 years were reported; 56% (34 of 61) of them had a history of household contact [10]. Through February 7, 2020, although the data were incomplete, 285 pediatric cases of 34,546 (0.8%) confirmed patients were documented, and 71.2% (183 of 257) of infected children reported having household contact [11]. The youngest affected patient was a 36-hour-old newborn, who remains the youngest COVID-19 patient worldwide [12]. As of February 11, 2020, the Chinese Center for Disease Control and Prevention reported 44,672 laboratory-confirmed cases; 416 cases (0.9%) were aged 0–9 years, and 549 (1.2%) were aged 10–19 years; 1 death occurred in the 10–19 years age group, and fatality rate in this group was 0.18% [13]. According to a report from the WHO-China Joint Mission on COVID-19, the largest-scale report to date, 55,924 cases were laboratory-confirmed by February 20, 2020. The median patient age was 51 years (range, 2 days to 100 years; interquartile range, 39–63 years); 2.4% of the affected patients were children under 19 years of age. Among children, 2.5% were severely ill and 0.2% were critical [6].
By February 10, 2020, a total of 10,924 adult cases and 398 pediatric cases were confirmed in mainland China, excluding the Hubei Province. The rate of pediatric cases was 3.5% (398 of 11,322 [9]). In 6 northern Chinese provinces, 31 children were diagnosed with COVID-19 between January 15 and February 21, 2020. The median pediatric patient age was 7.1 years (range, 6 months to 17 years); 21 children (68%) had a history of contact with a confirmed adult patient, while 28 children (90%) had an infected family member [14].
Pediatric COVID-19 cases outside of China have been sporadically reported, but limited data are available. Two Malaysian boys aged 2 years and 11 years on January 25 [15], a German boy on January 31 [16], a Singaporean 6-month-old boy on February 5 [17,18], and a Vietnamese 3-month-old infant on February 11 [19] were reported. All of these cases involved exposure to an infected family member.
In Singapore, after an index case from China was first diagnosed with COVID-19 on January 23, 2020, the infection spread through community transmission. By March 11, 2020, 167 patients were confirmed to have COVID-19, 6 (3.6%) of whom were children who were 6 months, 1 year, 2 years, 5 years, 12 years, and 17 years of age. Three children were residents of Wuhan; 2 children had an infected family member, and 1 reported exposure to an adult patient [17]. In Italy, in which a rapid increase in confirmed COVID-19 cases ensued after the cluster cases on February 21, 2020, a total of 8,342 cases had been reported by March 9, 2020; 1.4% of cases were in children aged 0–18 years, among whom there were no fatalities [20]. In Australia, a total of 71 COVID-19 cases were confirmed by March 7, 2020; 2 cases were diagnosed in the 0–9 years age group and 2 in the 10–19 years age group; therefore, 5.6% of the confirmed cases were in the 0–19 years age group [21] (Table 1).
In Korea, a 10-year-old girl whose mother and uncle had been diagnosed with COVID-19 was the first pediatric case, reported on February 18, 2020 [22]. Thereafter, newspapers reported a 4-year-old on February 23 [23]. a 45-day-old infant on February 29 [24], and a 4-week-old newborn on March 8 [25]. The 4-year-old was in contact with an infected daycare teacher, while the 45-day-old and the 4-week-old each were in contact with an infected family member.
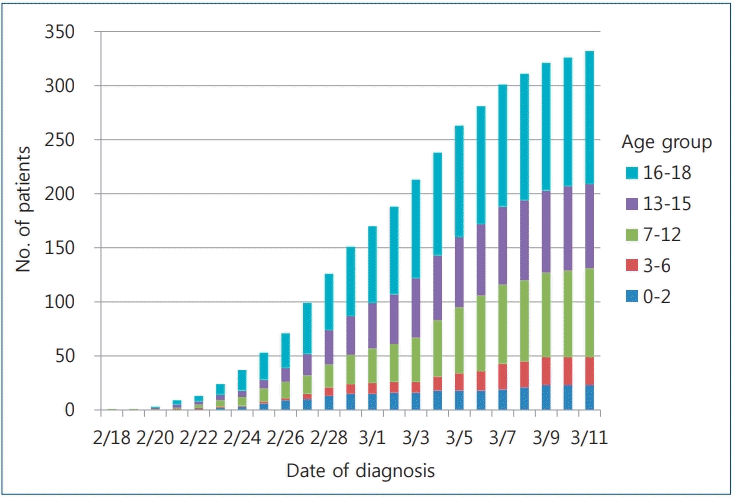
As of midnight March 11, 2020, a total of 7,755 patients had confirmed disease, of whom 75 (1.0%) were aged 0–9 years and 405 (5.2%) were aged 10–19 years. No fatalities have been reported in these age groups. The COVID-19 incidence rate in Korea is calculated as 15.0 per 100,000 in all ages, 1.8 per 100,000 in ages 0–9 years, and 8.2 per 100,000 in ages 10–19 years according to national population data in February 2020 (51,844,627 overall, 4,134,824 in the ages 0–9 years group, and 4,920,794 in the ages 10–19 years group [5,26]). As of March 11, 2020, according to the pediatric age group analysis by school years performed by the Korea Centers for Disease Control and Prevention, 23 affected children were 0–2 years, 26 were 3–6 years, 82 were 7–12 years, 78 were 13–15 years, and 123 were 16–18 years (Fig. 1) [27].
Clinical features in children
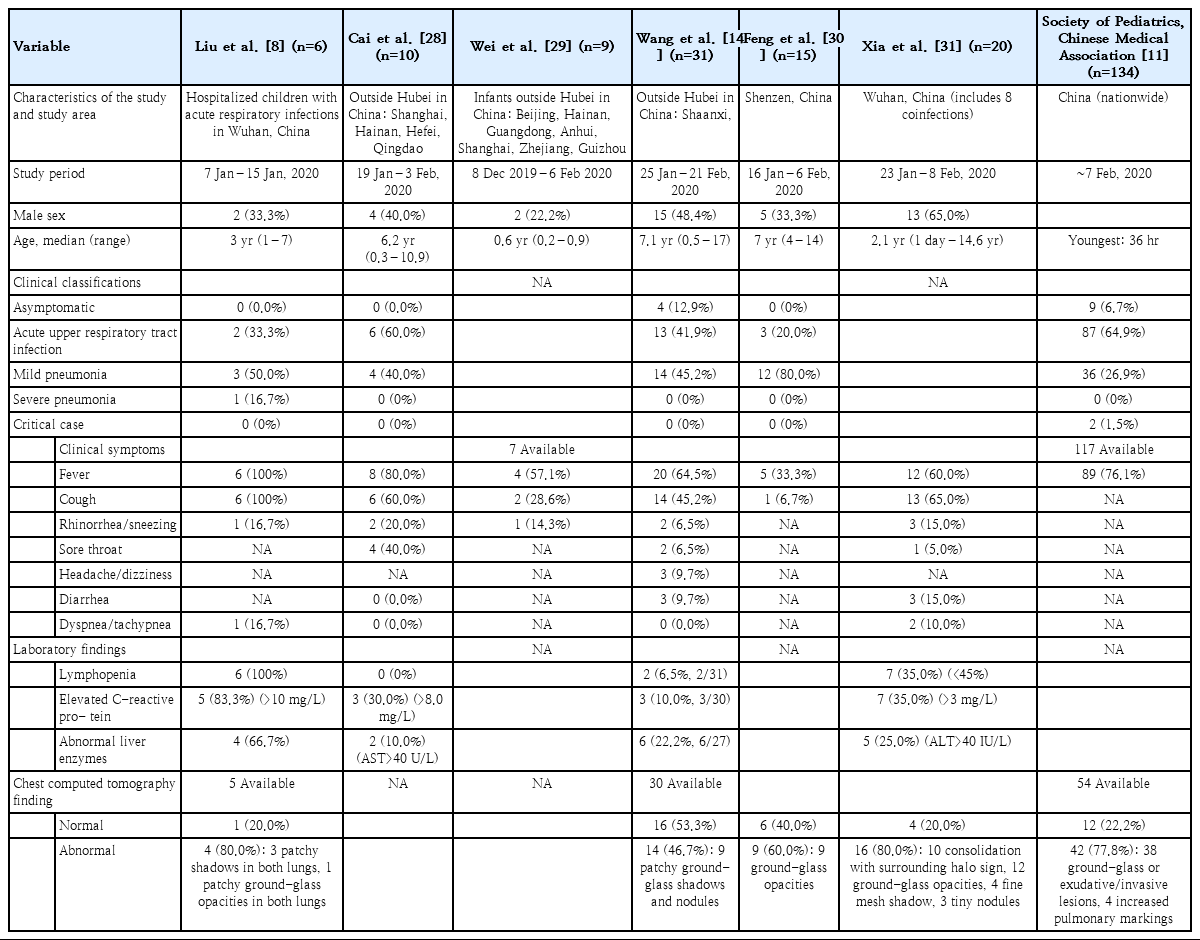
It is known that children with COVID-19 show milder symptoms than adults [11], but limited data exist on the burden of COVID-19 in children. A 10-year-old boy from Shenzhen, the first pediatric COVID-19 patient, was asymptomatic but displayed ground-glass opacity on an initial computed tomography (CT) scan [7]. Another study analyzed 10 laboratory-confirmed Chinese pediatric cases in Shanghai, Hainan, Hefei, and Quindao between January 19 and February 3, 2020; the median patient age was 74 months (range, 3–131 months). Fever was noted in 8, cough in 6, sore throat in 4, stuffy nose in 3, and rhinorrhea in 2 children. The fevers were 37.7°C–39.2°C and resolved within 24 hours. Patchy infiltrations were observed on the chest CT scans of 4 patients. None of the 10 patients required oxygen therapy [28]. Another study described 9 infants (age range, 1–11 months) in China outside of Hubei Province. Among the 7 infants who reported symptoms, 4 had fever, 2 had mild respiratory symptoms, and 1 was asymptomatic; no severe complications were noted [29].
In a study that enrolled 31 pediatric patients from 6 northern Chinese provinces between January 25 and February 21, 2020, 4 cases (12.9%) were asymptomatic, 13 (41.9%) were mildly symptomatic with normal radiography findings, 14 (45.2%) had pneumonia, and none had severe illness. Ten of the 31 patients (32.3%) complained of fever over 38°C, and 10 (32.3%) had a mild fever of 37.3°C–38°C. The fever lasted 1–9 days and subsided within 3 days in 15 patients (75%). Fourteen (45.2%) had a dry cough, 3 experienced fatigue, 3 had headache or dizziness, 2 had rhinorrhea, and 2 had a sore throat. One of the patients reported having a sore throat only, and 3 patients initially presented with diarrhea but without vomiting [14].
To date, 2 studies have described chest CT findings of pediatric patients in detail [30,31]. One study analyzed 15 pediatric COVID-19 cases admitted to a hospital in Shenzhen, China, between January 16 and February 6, 2020. The median patient age was 7 years (range, 4–14 years). At the time of diagnosis, 5 patients complained of fever, 1 had a cough, 1 had nasal congestion, and 8 (53.3%) were asymptomatic. The leukocyte count was decreased in 8 (53.3%) and within the normal range in 7 children. At the time of diagnosis, the chest CT scan revealed ground-glass opacity in 9 (60%) and appeared normal in 6 patients. A follow-up CT performed after 3–5 days showed the development of new inflammatory lesions with ground-glass opacity in 3 of 9 patients whose SARS-CoV-2 polymerase chain reaction (PCR) results continued to be positive. Among the 6 patients whose PCR results became negative, the follow-up chest CT findings improved in 2 [30].
A study reported 20 pediatric COVID-19 patients admitted to the Wuhan Children’s Hospital in China between January 23 and February 8, 2020. The median patient age was 2.1 years (range, 1 day to 14.6 years), and 3 neonates were included in the study. Thirteen patients complained of cough, 12 had a fever over 37.3°C, 3 had diarrhea, 3 had rhinorrhea, and 2 had tachypnea. Respiratory crackles were auscultated in 3 patients, chest retraction was observed in 1, and cyanosis was observed in 1. Most patients presented with signs of pneumonia. The leukocyte count was within the normal range in 14 (70%), decreased in 4 (20%), and increased in 2 patients (10%). The percentage of lymphocytes was decreased in 7 (35%) and increased in 3 patients (15%). C-reactive protein was increased in 9 (45%), and procalcitonin was increased in 16 patients (80%). Eight patients (40%) were coinfected with influenza A or B virus, Mycoplasma pneumoniae, respiratory syncytial virus, or cytomegalovirus. The initial chest CT scan showed unilateral pulmonary lesions in 6 (30%), bilateral pulmonary lesions in 10 (50%), and no abnor mality in 3 neonates and 1 child (4 of 20, 20%). All 20 child ren displayed subpleural lesions with localized inflammatory infiltra tion, 10 (50%) showed consolidation with surrounding halo sign, 12 (60%) showed ground-glass opacity, 4 (20%) showed a fine mesh shadow, and 3 (15%) showed tiny nodules [31].
The first severe pediatric case reported was a 13-month-old boy admitted to Wuhan Children’s Hospital on January 27, 2020. He presented with pneumonia, shock, acute respiratory distress, and renal failure at the time of admission. The patient had no known medical comorbidities; he had reportedly been treated at a local clinic for the past 6 days for intermittent diarrhea and vomiting but had no respiratory symptoms. He showed fever, dyspnea, and oliguria at the time of admission and received intensive care including intubation and mechanical ventilation. After treatment, he recovered gradually [32].
The largest-scale report to date on COVID-19 in children examin ed 134 patients whose clinical data were available among 285 pediatric cases diagnosed in China as of February 7, 2020. The most common symptoms were fever and cough; other clinical features included fatigue, myalgia, rhinorrhea, nasal congestion, sore throat, headache, dizziness, nausea, vomiting, abdominal pain, and diarrhea. The symptoms mostly resolved within a week. Among the 117 children whose body temperatures were recorded, 89 (76.1%) had a fever that lasted usually 1–2 days but up to 8 days. Complete blood counts were within the normal range in most patients; 2 showed a decreased leukocyte count, while 1 showed a slight decrease in lymphocyte count. C-reactive protein was normal or temporarily increased (>20 mg/L in 3 patients). When the 134 cases were classified by symptoms and chest radiology findings, 9 patients (6.7%) were asymptomatic with normal chest radiology findings, 87 (64.9%) had mild symptoms with normal chest radiology findings, 36 (26.9%, including 7 subclinical patients) had pneumonia, and 2 (1.5%) were critically ill (both of whom were mechanically ventilated). One of the critically ill patients was moderately malnourished and had a history of cardiac surgery for treating a congenital heart disease, while the other had hydronephrosis and a left renal stone. Among 54 patients for whom chest radiology data were available, 38 displayed ground-glass opacity or exudative/infiltrative lesions; of those, 7 were subclinical, 4 displayed increased pulmonary markings, and 12 showed no abnormalities [11].
In addition to the studies on clinical manifestations of COVID-19, one study analyzed pathogens retrieved from hospitalized children with respiratory infections during the early COVID-19 outbreak in Wuhan, China. Among the 366 children (≤16 years of age) admitted to 3 hospitals in Wuhan between January 7 and 15, 2020, 43 (11.7%) were infected with influenza A or B, while 6 (1.6%) were infected with SARS-CoV-2. Children with COVID-19 were aged 1–7 years. All 6 children had fever over 39°C (duration, 3–11 days; median, 6 days) and cough, and 4 of them had vomiting. The chest CT scans showed pneumonia in 4 patients. One patient, a 3-year-old in whom ground-glass opacity was noted on the chest CT scan, received intensive care including oxygen therapy. The median length of stay was 7.5 days (range, 5–13 days), and all patients recovered [8].
In summary, children with COVID-19 most commonly present with fever, cough, and fatigue along with nasal stuffiness, rhinorrhea, sputum, diarrhea, and headache. Patients may be afebrile or present with a mild fever; fever subsides within 1–2 weeks in most cases. Dyspnea and cyanosis can occur as the condition progresses, usually after 1 week of the disease and accompanied by systemic symptoms such as malaise or restless ness, poor feeding, poor appetite, and decreased activity. Pneumonia may develop; in some cases, it rapidly progresses and may cause respiratory failure that cannot be corrected by conventional oxygen therapy within 1–3 days. In severe cases, septic shock, metabolic acidosis, and irreversible bleeding and coagulation dysfunction might occur [33,34]. Ground-glass opacity and subpleural lesions were commonly observed on the chest CT scans (Table 2) [30,31].
In Korea, a case report on the first COVID-19 pediatric patient is the only known academic publication in children to date. A 10-year-old girl presented with a mild fever (37.3°C) and scarce amount of sputum. She tested positive for SARS-CoV-2. On the 4th day after symptom onset, the chest CT scan showed patchy nodular consolidations accompanied by ground-glass opacity. She did not require antiviral therapy and recovered after supportive care [22].
Importance of managing children with COVID-19
Numerous family clustering cases of COVID-19 have been reported in China. Of 1,836 cases that occurred in Guangdong and Sichuan Provinces, 1,308 were related to 344 clusters, most (78%–85%) of which occurred in families. According to a preliminary study ongoing in Guangdong, China, the secondary attack rate within a household is estimated to be 3%–10% [6]. Children primarily contract the disease through household exposure, and 56%–90% of infected children had an infected family member [10,11,14,28].
Children are usually diagnosed with COVID-19 after an exposure to an infected adult within or outside of the family circle; however, the source of infection could not be identified in some cases, while the diagnosis of a child preceded that of an adult in others. A 3-month-old child with confirmed COVID-19 on January 26, 2020, in Hubei Province was hospitalized with fever and found to have mild pneumonia on the chest CT scan. At the time of diagnosis, his parents were asymptomatic with negative SARS-CoV-2 PCR results. After 7 days of hospitalization of the infant, his father complained of fever and fatigue, while his mother was asymptomatic; both of his parents showed signs of pneumonia on the chest CT scan and had positive SARS-CoV-2 PCR results. It was undetermined whether the incubation period was shorter in the infant than in the parents or the virus was transmitted from the infant to the parents [9,35]. In the first severe case of a 13-month-old who developed pneumonia, shock, acute respiratory distress, and renal failure, the source of infection could not be identified. His parents were not tested for SARS-CoV-2 [32].
Influenza surveillance during the first 2 weeks of January 2020 in Wuhan City, in which the COVID-19 outbreak originated, indicated that SARS-CoV-2 was detected in adults but not in children [6]. In an analysis of 365 COVID-19 cases confirmed till February 5, 2020, in Shenzhen, China, the pediatric infection rate rapidly increased from 2% (before January 24) to 13% (between January 25 and February 5) (P<0.001). The possible reasons for this might be that exposure to SARS-CoV-2 was lower in children in the early course of the outbreak. Otherwise, children were less likely to be tested because of their mild presenting symptoms and signs, especially in the setting of limited resources in the early phase of the outbreak [36].
Little is known about the duration of viral shedding in children, and the reported durations varied widely across patients and samples. A 6-month-old infant diagnosed in Singapore on February 4, 2020 was asymptomatic but tested positive for SARS-CoV-2 PCR on a nasopharyngeal sample; his mother previously had confirmed COVID-19 pneumonia. The blood PCR sample turned positive for SARS-CoV-2 on hospital day 2, when the patient had a fever of 38.5°C that resolved immediately. A series of daily nasopharyngeal samples for PCR revealed that viral load presented as a 1/cycle threshold that peaked at the time of diagnosis and decreased over time. Negative conversion was achieved on hospital day 17. Stool PCR on hospital day 2 was negative but turned positive on day 9 [18].
In a study that enrolled 10 Chinese children in Shanghai, Hainan, Hefei, and Qingdao, symptoms appeared at a median 6.5 days (range, 2–10 days) from the onset of illness in the index case. Patients tested positive for SARS-CoV-2 PCR on a nasopharyngeal or throat swab 4–48 hours after symptom onset, while negative conversion was seen at a median 12 days (range, 6–22 days). Prolonged shedding of viral RNA in the feces was observed for 18–30 days in 5 patients [28].
In summary, the transmission of SARS-CoV-2 in children primarily occurs through contact with adult patients, mainly through household exposure. In contrast, direct transmission from a child to an adult has not been reported to date. However, the observation of prolonged detection of viral RNA in nasopharyngeal/throat swabs and feces of pediatric patients suggests that children and adolescents may be transmitters in the community.
Considerations in pregnant women and newborns
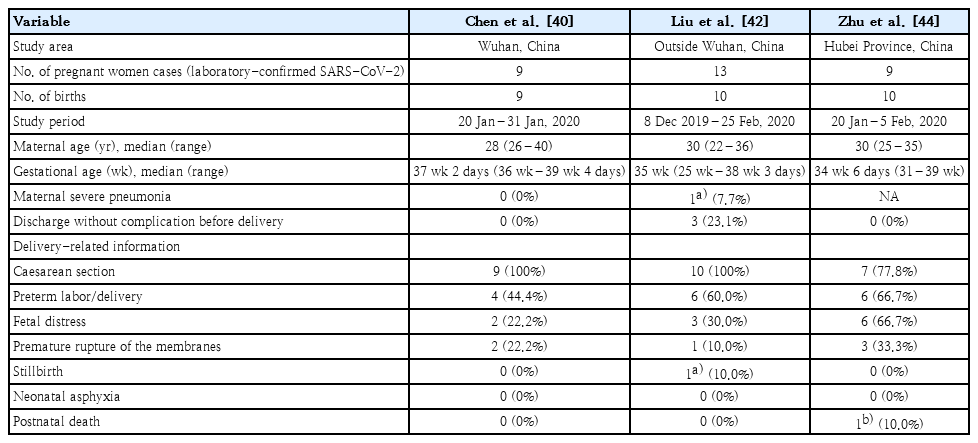
In the report of the WHO-China Joint Mission on COVID-19 patients diagnosed through February 20, 2020, pregnant women were not at higher risk for severe COVID-19. Of 147 (64 confirmed, 82 suspected, and 1 asymptomatic) preg nant women investigated, 8% had severe signs consisting of dyspnea and hypoxemia, while 1% was critical and requir ed intensive care [6]. However, pneumonia is one of the most common infections during pregnancy, as it may lead to pre mature rupture of the membranes, preterm labor, intrauterine growth retardation, and stillbirth [37-39]. Sporadic case reports on COVID-19 in pregnant women observed obstetric complications including stillbirth, preterm birth, and premature rupture of the membranes [40-43]. In a study of 9 pregnant women with COVID-19 diagnosed in Wuhan, China, from January 20 to 31, 2020, all women were infected at gestational age 36 weeks or later and delivered by caesarean section. Seven women complained of fever at the time of diagnosis, but none developed severe pneumonia that led to mechanical ventilation or death. Obstetrical complications included preterm deliveries in 4 and premature rupture of the membranes in 2; no cases of neonatal asphyxia or stillbirth were observed. From 6 patients, amniotic fluid, cord blood, breast milk, and neonatal throat swab samples were tested for SARS-CoV-2; all were negative [40]. A case report from Suzhou, China, described a pregnant woman at 30-week gestation. She was diagnosed with COVID-19 pneumonia on February 6, 2020 and delivered a 1.83-kg preterm baby via emergency caesarean section due to fetal distress. SARS-CoV-2 PCR of the amniotic fluid, placenta, cord blood, neonatal gastric juice, and neonatal throat swab was negative [41]. A study analyz ing 13 pregnant women with COVID-19 from December 8, 2019 to February 25, 2020 in mainland China outside of Wuhan included 2 women in their second trimester. Ten women had fever, 3 had dyspnea, and 1 was asymptomatic. Of the 13 women, 3 (23%) recovered and were discharged with uncom plicated ongoing pregnancy; 10 delivered by caesarean section. Five women underwent emergency caesarean section due to fetal distress (3 cases), premature rupture of membrane (1 case), and stillbirth (1 case). Six women had preterm labor. At 34-week gestation, one of the women developed acute respiratory distress syndrome and multiple organ failure requiring extra corporeal membrane oxygenation support; her baby was still birth [42].
In a report of 10 neonates born to 9 mothers with COVID-19 in Hubei, China, between January 20 and February 5, 2020, intrauterine distress was observed in 6 and premature rupture of the membranes was observed in 3. Six infants were born pre mature, 2 were small for gestational age, and 1 was large for gestational age. Clinical features of the newborns included shortness of breath (6 cases) and fever (2 cases). Two newborns developed thrombocytopenia complicated by abnormal liver function. One of the newborns was delivered at 34 weeks and 5 days’ gestation, developed shock and multiple organ failure on day 8, and died on day 9. Nine of the newborns underwent SARS-CoV-2 PCR testing on days 1–9; all were negative [44]. To date, there have been no reports of the vertical transmission of SARS-CoV-2. However, existing reports are incomplete because they included small sample sizes, primarily dealt with COVID-19 occurring in the third trimester, and not all pregnant women underwent SARS-CoV-2 PCR testing. Continuous monitoring is needed to assess the possibility of mother-to-child transmission.
To date, 3 cases of confirmed COVID-19 in neonates have been reported in Wuhan, China [11,12,31,45]. A neonate was diagnosed with COVID-19 at 36 hours after birth. His mother was at 40 weeks' gestation and developed a fever on February 1, 2020. The chest CT scan displayed ground-glass opacities suggestive of viral pneumonia. An emergency caesarean section was performed the same day and the newborn did not have contact with the mother after birth. The mother’s SARS-CoV-2 PCR result was positive the next day. A pharyngeal swab collected from the newborn at 36 hours after birth later tested positive for SARS-CoV-2. However, the cord blood, placenta, and breast milk were negative for SARS-CoV-2. The newborn was afebrile with no coughing or vomiting; however, the chest CT scan showed a nodular shadow under the pleura on day 6. The follow-up chest CT scan showed small patchy shadows on day 12 that improved on day 17. SARS-CoV-2 PCR conducted of pharyngeal and anal swabs on day 17 were negative and he was discharged on day 18 [12]. Another 5-day-old neonate with a fever was diagnosed with COVID-19, and his mother was diagnosed with COVID-19 [11]. A 17-day-old newborn was hospitalized on February 5, 2020 with a 1-week history of sneezing and vomiting. His parents were diagnosed with COVID-19 3 days prior. His chest CT scan showed increased pulmonary markings; all symp toms resolved by hospitalization day 7 [45]. A small number of neonatal COVID-19 have been reported to date, and the infected neonates had mild or no symptoms. However, current data support that neonates are susceptible to COVID-19; therefore, special precautions including hand hygiene are required of individuals in close contact with newborns (Table 3).
Conclusion
COVID-19 is a novel infectious disease that was recently declared a pandemic. It is rapidly spreading worldwide, infecting and killing thousands of people. Limited information is available on this previously unknown coronavirus that has been around for less than 3 months. It is known that children comprise a small fraction of COVID-19 cases, and their symptoms are often mild. However, some pediatric cases may progress to severe disease, and initial atypical presentations may delay the diagnosis of COVID-19, leading to unfavorable outcomes. It is worth mentioning that newborns are susceptible to this disease and viruses are detected for a prolonged period; therefore, newborns might play a role in community transmission. There is currently no standard of management or prevention. Further investigation is needed to assess the reason for differences in clinical features of COVID-19 by age, evaluate the role of children in community transmission, and develop treatment and vaccines for the disease. We hope that current and future research on COVID-19 can answer these questions and look forward to wisely managing this crisis for mankind.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We are grateful for the support of the members of the Korean Society of Pediatric Infectious Diseases, the Committee on Infectious Diseases of the Korean Pediatric Society, and the Korea Centers for Disease Control and Prevention.
This review article is published jointly by the Clinical and Experimental Pediatrics and the Pediatric Infection and Vaccine.