Pediatric heart transplantation: how to manage problems affecting long-term outcomes?
Article information
Abstract
Since the initial International Society of Heart Lung Transplantation registry was published in 1982, the number of pediatric heart transplantations has increased markedly, reaching a steady state of 500–550 transplantation annually and occupying up to 10% of total heart transplantations. Heart transplantation is considered an established therapeutic option for patients with end-stage heart disease. The long-term outcomes of pediatric heart transplantations were comparable to those of adults. Issues affecting long-term outcomes include acute cellular rejection, antibody-mediated rejection, cardiac allograft vasculopathy, infection, prolonged renal dysfunction, and malignancies such as posttransplant lymphoproliferative disorder. This article focuses on medical issues before pediatric heart transplantation, according to the Korean Network of Organ Sharing registry and as well as major problems such as graft rejection and cardiac allograft vasculopathy. To reduce graft failure rate and improve long-term outcomes, meticulous monitoring for rejection and medication compliance are also important, especially in adolescents.
Introduction
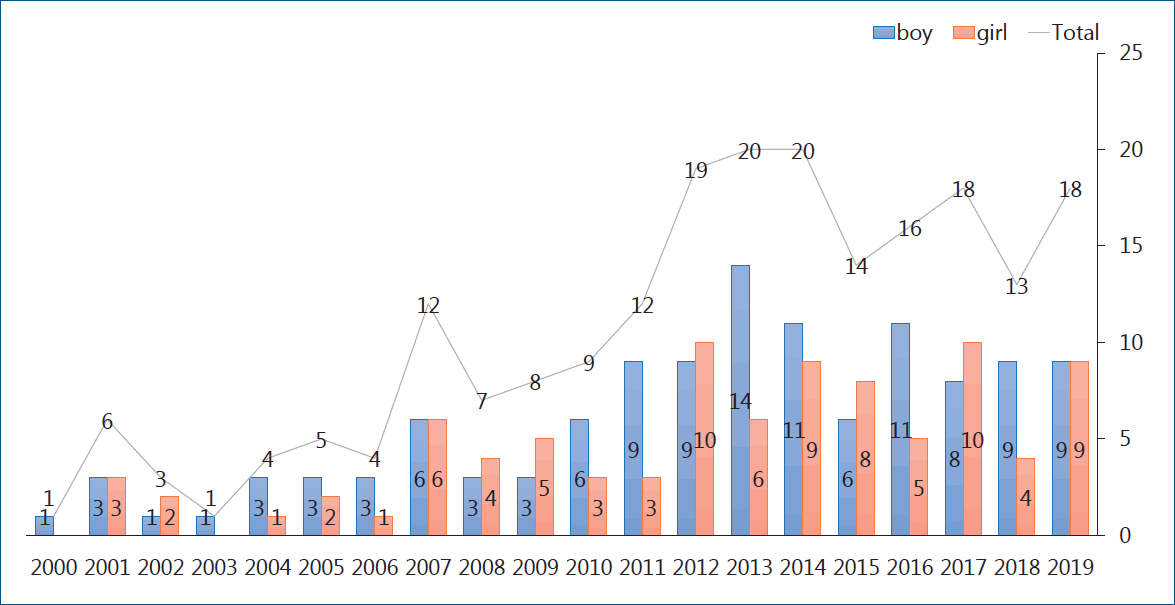
Heart transplantation is considered the definite therapeutic option for patients with end-stage heart disease. The first pediatric heart transplantation was performed in 1968 by Kantrowitz et al. [1] in a 3-week-old patient with tricuspid atresia from anencephalic infant donor. The annual report from the International Society of Heart Lung Transplantation (ISHLT) stated that; cases of pediatric heart transplantation have markedly increased since publication of the first registry report in 1982 and that 550 pediatric patients underwent heart transplantation annually and steadily [2]. Generally speaking, pediatric heart transplantation comprises about 10% of total cases of heart transplantation. In a 2016 pediatric heart transplantation report of a total of 12,091 cases in 1982–2014, congenital heart disease (CHD) was the most common underlying heart disease in the infant group, whereas dilated cardiomyopathy (DCM) and retransplantation are more common in older age groups [3]. In Korea, the first pediatric heart transplantation was performed in 1997 in a 17-year-old adolescent patient with DCM on the mechanical circulatory support (MCS). Data from Korean Network of Organ Sharing (KONOS) showed that the number of heart transplantations has increased and steadily by 10–15 cases annually, thus a total of 190 heart transplantations have been performed to date (Fig. 1). The long-term outcomes of pediatric heart transplant recipients are reportedly comparable to those of adults. Using the long-term follow-up data of the registry, we analyzed patient survival, complications, morbidities, and mortality. The overall actuarial survival rate is 83% at 1 year after transplantation, 73% at 5 years, 61% at 10 years, 43% at 20 years, and 35% at 25 years [3]. In the recent 22nd pediatric heart transplantation report - 2019, the overall median survival was more than 18 years, with the longest survival (median, 24.5 years) being of infant transplant recipients, and the shortest survival (median, 14.3 years) being of 11–17 years of age. Overall, recipients with DCM have superior survival to those with CHD or those undergoing retransplantation. Importantly, recipients supported by a ventricular assist device (VAD) or total artificial heart had similar survival rate to those without MCS, but the use of extracorporeal membrane oxygenation (ECMO) as a bridge to transplant continued to be associated with decreased survival [4]. This article reports on the pretransplant evaluation immunosuppressive regimen and major long-term problems after transplantation such as graft rejections, acute cellular rejection (ACR), antibody-mediated rejection (AMR), primary graft failure, and cardiac allograft vasculopathy (CAV).
Recipient’s evaluation and listing
Generally accepted indications for heart transplantation are circulatory failure that is refractory to optimal medical and surgical treatment, poor short-term prognosis, and unacceptable quality of life. The decision to proceed with an evaluation and listing for heart transplantation is based on an estimated life expectancy of less than 1 year. Contraindications include conditions such as active infection, especially hepatitis C viral infection, progressive systemic disease, severe metabolic disease, multiple other severe congenital anomalies, multisystem organ failure, active malignancy, or a cognitive or behavioral disability of the parents or health-care provider that interferes with posttransplant compliance and positive human immunodeficiency virus serology. However, patients with a well-controlled active infection, or malignancy are considered relative contraindications. Controversy persists about patients with chromosomal anomalies. The ability to achieve a good quality of life and an enhanced life expectancy are considered upon listing these patients. Multidisciplinary approaches are recommended to decide a patient’s listing for heart transplantation. Recipients must be evaluated prior to being added to the KONOS list (Table 1) [5]. A hemodynamic evaluation is needed. Prolonged end-stage heart failure resulted in pulmonary venous hypertension and progressed to pulmonary arterial hypertension. An elevated indexed pulmonary vascular resistance (PVRi) is a recognized risk factor for increased early mortality after heart transplantation. The measurement of PVRi and transpulmonary gradient (TPG) is mandatory for listing decision-making. Adults with a TPG more than 15 mmHg or a PVRi more than 6 wood units are considered unacceptable candidates, but children are considered acceptable even with a TPG more than 15 mmHg when the PVRi is less than 10 wood units. A value of more than 10 wood units is considered an absolute contraindication for pediatric heart transplantation. When patient’s baseline level is elevated beyond these limits, we perform an acute vasoreactivity (AVR) test. If patients responded to the AVR test, they were considered acceptable candidates. Each patient’s immunization history is also important. Patients are required to receive immunizations, especially hepatitis B, pneumococcal vaccine, and influenza before transplantation. Donor-recipient size compatibility and cross-match are important. The acceptable ratio of the donor/recipient weight is 1.5 in adults, but 2.5 in children and 3–4 in newborns and infants. An oversized donor heart may give rise to a postoperative syndrome characterized by a high output state such as systemic hypertension, increased intracranial pressure and mental status change [6]. A recent study reported accepting a maximum donor-recipient weight ratio of 3 in pediatric heart transplantation [7]. An undersized donor heart is more harmful than an oversized donor, therefore, according to the ISHLT guideline, the use of a heart from a donor whose body weight is no greater than 30% less than that of the recipient is uniformly safe. In some transplantation centers, height, body mass index, or body surface area are used. Recently, pediatric heart mass, an estimate that incorporates height, weight, age, and sex has been proposed as the optimal metric for size matching in adult, but not pediatric heart transplantation. Next, we should check recipient’s blood test for panel reactive antibody (PRA) level and cross-match. Recipients undergo PRA testing for the presence of anti-human leukocyte antigen (HLA) antibodies by complement-dependent cytotoxicity or enzyme-linked immunosorbent assay for a panel of 56 class I and 32 class II HLA. A high PRA (>10%) is associated with increased risks of rejection, CAV, and death. Although prospective cross-match abrogates the risk of posttransplant mortality, it may contribute to higher pretransplant attrition due to longer waitlist times [8-10]. HLA tying is not mandatory in heart transplantation.
Immunosuppression
T-cell activation requires 2 signals such as T-cell receptor by a specific antigen and costimulation not associated with a specific antigen. Activated T-cell gives rise to T-cell proliferation and lymphokine secretion, especially interleukin 2 (IL-2), which binds to interleukin 2 receptor (IL-2R) on the activated T-cell surface and causes T-cell activation. The induction of immune suppressive management is designed to reduce the incidence of early rejection and delay maintenance immunosuppressive therapy, which prevents acute kidney injury. Polyclonal antibodies such as antilymphocyte globulin (ALG), antithymocyte globulin (ATG) or an IL-2R blocker such as basiliximab, daclizumab are proved by the U.S. Food and Drug Administration as an induction regimen. There was no statistically significant difference in survival rate between no-induction and induction regimen groups [11]. The induction regimen is recommended in recipients with a calculated PRA (cPRA) >50% and CHD. ALG/ATG represents the most common use in nearly 50% versus an IL-2R blocker in just over 20%. ALG/ATG is more potent than the IL-2R blocker. Routine use of an induction regimen with ALG/ATG is indicated when a complete steroid avoidance regimen is planned after pediatric heart transplantation. Methyl prednisolone 10 mg/kg is used immediate after the release of aortic cross-clamping in the operating room, and then 2 mg/kg/dose intravenously every 8 hours (3 doses) for the first 24 hours, and 0.5 mg/kg twice enteric or intravenously for the next 24 hours [12]. Maintenance therapy includes steroids, calcineurin inhibitor (CNI), antimetabolites or mammalian target of rapamycin (m-TOR) inhibitor. Maintenance therapy should include CNI in all pediatric recipients (class 1C) [13]. CNI includes the antibiotics cyclosporine-A (CSA), which was isolated from the fungus Tolypocladium inflatumgams and tacrolimus (FK-506), a naturally occurring macrolide. Their efficacies are equivalent, but the trend of their usage is changing from CSA to tacrolimus since 2010. Major significant side effects of CNI include systemic hypertension, hyperlipidemia, and posttransplantation diabetes mellitus (PTDM). CSA causes systemic hypertension more frequently than tacrolimus, which, in contrast, causes PTDM more frequently. Initial triple combination such as a steroid, CNI and an antimetablite, such as mycophenolate mofetil (MMF) is the mainstay of maintenance therapy. MMF, Sirolimus or everolimus as an m-TOR inhibitor should be included to reduce CAV onset and progression. Combination therapy with CNI and an m-TOR inhibitor shows synergistic effect. Blood level monitoring of CNI is important to reducing side effects. Steroids have potent immunosuppressive and anti-inflammatory effects, but many adverse effects such as moon face, truncal obesity, acne, avascular necrosis, osteoporosis, and growth failure. A steroid free protocol is advisable and should be mandatory in pediatric patients to prevent growth retardation, insulin dependent diabetes mellitus, serious infection, metabolic disorder, and obesity. Steroid withdrawal can be successfully achieved in 50%–80% of cases subjected to late (6–12 months) better than early withdrawal (3–6 months) [14].
Cause of death
To improve the long-term outcomes of infant and pediatric heart transplant recipients, thorough evaluations and death preventive measure are needed. Causes of death were categorized by standard clinical and pathological criteria (Table 2) [15]. Death was also categorized according to the period after transplantation into early (≤1 month after transplantation), intermediate (1 month to 1 year), late (1 year to 5 years), and very late (≥5 years). The majority of early deaths are due to acute graft dysfunction and technical issues, intermediate death due to acute rejection and infection, late death due to acute rejection and CAV, and very late death due to CAV and malignancy, especially posttransplant lymphoproliferative disease. Acute rejection was the single most important cause of death and resulted in a significant number of sudden unexpected death [15]. Primary graft dysfunction (PGD) is defined as the development of left or biventricular dysfunction shortly after heart transplantation without any identifiable causes such as acute rejection, pulmonary hypertension, or surgical complications. Patients with severe PGD required MCS (VAD or ECMO). The incidence of severe PGD in pediatric heart transplant recipients is reportedly 4.7%, which remains an important clinical morbidity and is associated with high mortality [16].
Rejection
Rejection is an adaptive immune response mediated through T-cell and humoral immune mechanisms targeting myocytes or vascular endothelial cells. Rejection is initiated by the presentation of donor antigens as an antigen-presenting cell to the recipient’s T-cells. Despite improved management strategies for rejection, it still limits recipient survival and quality of life.
Diagnosis of rejection
Rejection surveillance by periodic endomyocardial biopsy (EMB), which remains the gold-standard method for detecting rejection, is important. The grading system for rejection severity was introduced in 1990 and revised through the 2004-ISHLT working formulations (Tables 3, 4) [17]. However interpretation of pathologic specimens from cases of acute rejection vary among pathologists despite established criteria, and EMB is aggressive and invasive; the need to collect numerous consecutive biopsies, especially within the first several months of cardiac transplantation, may result in serious complications such as severe tricuspid regurgitation and coronary-to-right ventricular fistulae [18,19]. In children, especially infants, invasive EMB can be replaced by echocardiographic examination [20,21]. Besides EMB or echocardiography, cardiac allograft rejection is suspected when the symptoms and signs of heart failure or new-onset arrhythmias are seen or by cardiac imaging such as cardiac computed tomography or magnetic resonance, numerous biomarkers, and AlloMap results [22-26]. Many different biomarkers such as cardiac troponin I or T, brain-type natriuretic peptide (BNP), N-terminal pro-BNP (NT-proBNP), soluble suppressor of tumorigenicity-2 (sST-2), inflammatory biomarker (C-reactive protein [CRP], tumor necrosis factor alpha, IL-6) have been introduced through single-center studies or small multicenter cohort studies and clinical trials. Among these inflammatory biomarkers, highly sensitive CRP (hs-CRP) >0.78 mg/dL was the most useful parameter of ACR during the first-year posttransplantation [27]. Furthermore, serum sST-2 is elevated (sST-2 >600 pg/mL) in heart transplant rejection, ACR, and/or AMR, and decreases with rejection management [28]. AlloMap, peripheral blood mononuclear cell profiling, is the most rigorously evaluated screening test for transplant recipients above 15 years of age, but was not developed or validated for children [29,30]. In the new genomic technique, quantitation of donor-derived cell-free DNA (dd-cf DNA) (%), a sensitive noninvasive marker of acute rejection after heart transplantation, and standardized dd-cf DNA testing, is conducted with plasma dd-cf DNA quantification using targeted amplification and the sequencing of a single nucleotide polymorphism panel. This technique had 44% sensitivity to detect rejection and a 97% negative predictive value for dd-cf DNA being elevated 3-fold for a risk of AMR. The reported test performance characteristics will guide the next stage of clinical utility studies of the dd-cf DNA assay [31,32].
Management of rejection
Acute rejection with hemodynamic compromise was defined as an episode of acute graft rejection for the requirement of intravenous inotropic support while undergoing rejection treatment. This rejection is associated with a mortality rate as high as 30%–50% in pediatric heart transplant recipients [33,34]. Acute rejection with hemodynamic compromise requires management regardless of rejection type. Initial rescue therapy is methyl prednisolone pulse therapy for 3 days and proceeded to high-dose prednisolone (1 mg/kg/day), then tapered for 2 weeks. If not improved, ATG as an anti-T-cell antibody for 7–10 days is needed in combination with intravenous immunoglobulin (IVIG). If humoral rejection is suspected, plasmapheresis and rituximab may be required. If graft failure continues irrespective of antirejection therapies, MCS is necessary and retransplantation must be sought. A report from a Children’s Hospital of Colorado group demonstrated that overall graft survival of hemodynamically compromised graft rejection was worse (41%) and not statistically different between the higher and lower inotropic groups despite of better short-term outcomes of the lower inotropic group. The long-term survival of the lower inotropic group became worsened, which is related to worsening or aggressive transplant coronary artery disease [35].
Acute cellular rejection
ACR has been well described and studied. Its grading system also well established, so management is decided by pathologic grading system score. In the revised grade 1 (1R) rejection, standard immunosuppressive management was continued in the present manner. In 2R rejection, high-dose oral prednisolone (1 mg/kg/day) is recommended and tapered to the present level or discontinued for 2 weeks, and continued the present immunosuppressive regimen with a dose increment or change to another regimen, but in 3R rejection, the rescue therapy is started [13]. Response to therapy is confirmed by follow-up EMB at 1–2 weeks after the initiation of management. If initial rescue therapy fails, ATG is recommended for 7–10 days. The cumulative effect of rejection ≥2R is associated with CAV onset [36].
Antibody-mediated rejection
AMR was first described by Herskowitz et al. [37] as a pathologic diagnosis of arteriolar vasculitis. Hammond continued to provide the initial immunohistochemical evidence that involved antibody deposition with subsequent complement activation. The ISHLT reviewed the pathologic changes, identified by a blueprint capillary endothelial changes, as macrophages and neutrophil infiltration, and interstitial edema, the linear accumulation of immunoglobulin and complement component C4d along the capillary endothelium [38]. Compared to ACR, AMR has been variably defined and poorly understood because standardized scheme for diagnosis and treatment remain contentious. By the ISHLT 2010 working formulation proposal, a new pathologic AMR (pAMR) grading system was introduced (Table 5). AMR is defined as either EMB consistent with pAMR or a rejection event based on immunotherapy augmentation direct against antibody production. Mixed rejection consisting of both ACR and AMR has been well documented and also has shown an increased incidence of AMR with more severe ACR [39]. In Pediatric Heart Transplant Society (PHTS) database report, the rate of mixed rejection of AMR are 23% with 2R-ACR versus 7% with 3R-ACR. AMR has been reported in up to 20% after heart transplantation and its risk factors were identified such as female recipients, multiparity, blood transfusion (particularly platelets), positive crossmatch, allosensitization, VAD, and previous surgery for CHD, especially with homograft use. In pediatric heart transplantation, risk factors for AMR include a diagnosis of CHD, age at transplantation, allosensitization, positive cross-match, presence of severe ACR, and use of maintenance steroid [40]. Symptomatic AMR is associated with a higher risk of cardiac dysfunction and hemodynamic compromise at presentation, CAV, and poor survival outcomes. Initial rescue therapy is mandatory in cases of symptomatic AMR, and it is necessary to check the pathologic diagnoses including immunohistopathology and check circulating donor specific antibody (DSA). Circulating DSA does not induce rejection; it only sensitizes recipient’s B cells to the donor heart, which can target antigens located on the transplanted heart and cause graft dysfunction through a complex cascade. AMR is a pathologic diagnosis dependent on immunohistopathology but independent of antibody status [41]. If AMR is confirmed by EMB in patients with cardiac allograft dysfunction, immunotherapy augmentation direct to antibody production is crucial. However, the clinical significance of asymptomatic AMR is controversial, the incidence of AMR can be underestimated. Utah Transplantation Affiliated Hospital (UTAH)-Cardiac Transplant Program database has kept details record of all EMB specimens collected from the entire transplanted population and histologic and immunofluorescence findings were recorded separately in the pathologic databases. The UTAH group report on the incidence and course of asymptomatic AMR on routine surveillance of EMB, the incidence of asymptomatic AMR is higher in the first-year posttransplant than later posttransplant groups (13.6% vs. 5.2%) and likelihood of resolution is lower on follow-up EMB, especially in more severe grade of AMR. These findings may be helpful in the planning of future studies to test whether therapeutic intervention on asymptomatic AMR favorably impacts outcome [42]. They reported that pAMR2 or higher was present 18% of pediatric heart transplant recipients, while pAMR3 was associated with worse cardiovascular outcomes [43].
Should asymptomatic AMR be treated? Wu et al. [44] published a study comparing 5-year actuarial survival freedom from CAV in 21 untreated asymptomatic AMR patients, 22 treated AMR with left ventricular (LV) dysfunction patients, and a matched control group (86 contemporaneous patients without AMR) and concluded that survival was comparable but CAV was more likely to develop in untreated asymptomatic AMR than the control group, even trending to do worse than the treated symptomatic AMR group. Testing for DSA should be performed when routine EMB including surveillance of AMR and, DSA was used annual follow-up.
The management of AMR involves the same strategies as its desensitization and prevention. Desensitization and reducing antibody production and/or circulating HLA antibody is essential to most strategies, and while others focus on mitigating the effects of circulating antibody. IVIG was used for the desensitization and treatment of AMR, and the mechanism of action included inhibition of antibody production, neutralization, elimination of antibody and inhibition and reduction of complement-mediated injury through blocking membrane attack complex formation on endothelial cells [45]. To remove circulating antibodies, plasmapheresis or a protein A-immunoadsorption column, derived from Staphylococcus aureus, have been used. To decrease antibody production, rituximab, a chimeric monoclonal antibody against CD20 B cells, bortezomib, a proteosomal inhibitor that blocks plasma cells, the primary source of HLA antibody, have been used. Eculizumab, a humanized anti-C5 monoclonal antibody that blocks activation of the terminal complement cascades, is introduced for the management of atypical hemolytic uremic syndrome in patients with renal transplantation [46], but anecdotal cases are reported in pediatric heart transplantation [47]. An open-label, investigator-initiated pilot study on the effect of eculizumab is currently ongoing at Cedars-Sinai Medical Center (DUET;NCT02013037). In this trial, eculizumab was administered to de novo heart transplant recipients with a cPRA of greater than 70%. The primary end-point was the incidence of pAMR with LV dysfunction in the first 6 months after transplantation [48]. According to data from the PHTS database, IVIG is the most common individual therapy (58%), combination therapy with plasmapheresis and/or rituximab and/or bortezomib were also introduced, and the group reported that the 1- and 3-year freedom rates from AMR after heart transplantation were 88% and 82%, respectively and the 1-, and 3-year survival rates after the initial AMR diagnosis 88% and 77%, respectively, and short term and graft survivals were worse for those treated with isolate AMR than those with mixed rejection and without AMR groups [37]. Reasonable long-term outcomes can be achieved after desensitization with a combination of plasmapheresis and/or rituximab and/or IVIG reportedly with 5-year freedom from CAV and death [49]. CPRA stands for the high-level antibody against common HLA antigens, indicating a lower ability to find a suitable donor heart. If the cPRA is above 50%–70%, desensitization therapy may be used to reduce antibody levels before transplantation [48]. DSA is measured by the single antigen bead test. For DSA testing, sera were screened HLA antigen following the manufacturer’s suggested protocol using a panel of up to 100 different color-coded beads each, coded with purified single HLA class I and class II antigens (LABScreen Single Antigen Beads: One Lamda, Inc., Canoga Park, CA, USA), using LABScan 100 flow analyzer (Luminex Corp., Austin, TX, USA). Measured DSA is expressed as mean fluorescent intensity (MFI), which means strength of binding to antigen. The higher level of MFI, the stronger the affinity to bind specific HLA antigen. The UTAH group analyzed the data of pediatric heart transplant recipient with EMB, paired with DSA testing, they concluded that the correlation of DSA-MFI strength with higher AMR biopsy-grade and the trend toward differences in longer term cardiovascular outcomes, provide the evidence for routine DSA monitoring after pediatric heart transplantation [50].
Cardiac allograft vasculopathy
CAV is major cause of morbidity and mortality beyond the first year after transplantation. According to the ISHLT registry data, 60% of pediatric recipients are free of CAV at 11 years post-heart transplantation. CAV is observed in adult and pediatric patients, but the clinical findings, predictors, role of ISHLT grading system for CAV severity, and the prognostication of outcomes of CAV in pediatric patients have not been well defined. A consensus statement from the ISHLT working formulation-2010 provided the foundation for the recommended nomenclature of CAV (Table 6) [51,52]. CAV is characterized by heterogenous intimal proliferation, medial wall thickening, and progressive luminal stenosis. Because a donor heart coronary artery evaluation was unavailable at the donation step, a baseline evaluation of donor heart by conventional coronary angiography and/or intravascular ultrasonography (IVUS) after transplantation is needed in acceptable pediatric recipients at the first EMB. A Follow-up evaluation is mandatory to detecting CAV at the first-year posttransplantation. IVUS can be surrogated coronary angiography in case of subclinical CAV. Serial assessments of IVUS study are essential to distinguishing early CAV from donor-transmitted conventional atherosclerosis. Maximal intimal thickness (>0.3 mm) is positive for intimal hyperplasia, which may represent an oversimplification of the disease process involved in CAV, but remains one of the best available surrogate markers for predicting CAV outcomes. If positive in intimal hyperplasia, regular coronary angiography and/or IVUS is recommended every 6 or 12 months, but can be substituted to the noninvasive image modality in pediatric recipients. Noninvasive imaging technique including myocardial perfusion scan with Tc-99m sestamibi, dobutamine stress echocardiography, and multidetector computed tomography (MDCT). Clinical manifestations of CAV vary. The denervated donor heart gains reinnervation, which is often incomplete, in up to 40% of patients during the first-year posttransplantation. Clinical symptoms of ischemia or infarction with CAV may or may not include chest pain, or atypical chest pain. Patient with complex symptoms, such as the combination of chest pain, abdominal pain and/or arm pain without other causes, were likely to have CAV. Sudden death or resuscitated sudden death occurred in 68% of patients with pain symptoms [53,54]. Previous adult and pediatric studies have shown that older donor age, male sex, and hypertension are associated with a higher risk of CAV and that recipient risk factors include male sex, older age, early severe rejection, increased number of rejection episodes, CMV infection, diabetes, hypertension, hyperlipidemia, long-term steroid use, and obesity. The PHTS group recently reported CAV risk factors including older recipient, donor age, and early rejection (≥2 rejections within 1 year after transplant), while the ISHLT reported that the only CAV risk factor was recipient age (≥11 years) [55,56]. A report from the Organ Procurement and Transplantation Network/United Network of Organ Sharing database of pediatric heart transplantation showed that the estimated incidence of CAV by the Kaplan-Meier method was 13% at 5 years, 25% at 10 years, and 54% at 15 years after transplant, respectively, and that older recipient and donor age, black recipient, donor cigarette use, and retransplantation were highly associated with a shorter time from transplantation to CAV development in pediatric heart transplant recipients. This group recommended that the higher-risk recipients may be monitored more closely in terms of other modifiable traditional risk factors, thus potentially reducing CAV incidence/severity and increasing graft survival [57]. Schumacher et al. [58] reviewed the pathogenesis of CAV, including constitutional factors such as genetic and metabolic predisposition, ischemic reperfusion injury of the donor heart, immune response from various factors (immunosuppressants, and infection, especially Epstein-Barr virus, cytomegalo virus, and parvovirus). CAV remains the leading challenge for long-term graft survival, but its pathogenetic mechanisms are still incomplete, which has limited the development of therapies. CAV progression has been traditionally managed with m-TOR inhibitors such as sirolimus, and everolimus, which inhibit smooth muscle cell proliferation [59]. To prevent CAV development or progression, statins are a uniquely effective medicine, whereas aspirin and β-blockers are ineffective [60]. Angiotensin-converting enzyme inhibitor used seemed promising to plaque regression and positive vascular remodeling [61]. More importantly, close monitoring is mandatory such as regular MDCT and echocardiography screenings and biomarkers such as BNP, sST2 and hs-CRP, and DSA especially anti-class II antibody. Mehra et al. [62] reported that patients with a BNP>250 pg/mL had a 13.6-fold increased risk of death secondary to graft failure, sudden death, or CAV. Claudius et al. [63] reported that a BNP>100 pg/mL in pediatric patients was associated with graft pathology including CAV. An elevated BNP without rejection suggests higher index of CAV [63,64]. There are no pediatric data to support the use of either troponin I or hs-CRP to aid in the detection of CAV. Tissue Doppler imaging has been used to make the diagnosis of acute rejection episode and has been investigated as a tool in the detection of CAV [65]. The early assessment of LV global longitudinal strain (LVGLS) is a noninvasive predictor of 1-year mortality of heart transplant recipients [66]. Chronic vascular rejection, or CAV, is known to involve epicardial vessel and microvascular function, which results in perfusion abnormalities in the endocardium. Longitudinal muscle fibers of the left ventricle are mainly located in the endocardium, so LVGLS is affected by perfusion defects caused by CAV. Graft dysfunction is important for deciding the degree of CAV. Severe CAV involves allograft dysfunction (LVEF ≤45%, regional wall motion abnormalities, or restrictive physiology). Clemmensen et al. [67] reported that the LVGLS was significantly reduced according to CAV degree (CAV 0, -16.7%; CAV 1, -15.2%; CAV 2–3, -14.0%) and suggested LVGLS as a new method for monitoring graft function in relation to CAV. Buddhe et al. [68] reported that diastolic function parameters by spackle tracking echocardiography imaging better correlated with pulmonary capillary wedge pressure than traditional echocardiographic parameters, and that abnormalities of longitudinal systolic dysfunction may be common in pediatric heart transplant recipients without acute graft rejection. The cutoff value of LVGLS in pediatric heart transplant recipients is reportedly -18% to differentiate normal from abnormal. In general, the management of CAV involoves coronary revascularization procedures. In adults, percutaneous revascularization procedures have variable success rates with high restenosis rates and little impact on graft survival. In pediatric recipients, data from PHTS showed that revascularization procedures were performed in only 1% of patients including stenting (75%), and balloon angioplasty (25%), and the freedom from graft loss after the procedure was 89%, 75%, and 61% at 1, 3, and 12 months respectively [69]. Because of poor outcome after procedure, listing for retransplantation is mandatory. As mentioned above, an incomplete understanding of CAV pathogenesis has limited the development of therapies directed at process reversal or avoidance. Further studies, particularly in the pediatric heart transplant population, are needed to better elucidate the disease mechanisms and potential targets for further therapeutic intervention.
Conclusions
Rejection and CAV are major problems affecting long-term outcomes and graft survival. To improve graft survival, careful surveillance and early intervention for graft rejection are important. EMB is the gold-standard for detecting rejection, but other surveillance methods, such as echocardiography and various biomarkers, are surrogates. Hemodynamic compromise rejection involves urgent management with initial rescue therapy irrespective of rejection type. Asymptomatic AMR is more prevalent in the first-year posttransplant than later on. Therefore, routine EMB with histology, immunofluorescent examination, and DSA testing is mandatory. Like ACR, AMR has a severity grading system. We confirmed that asymptomatic AMR that had progressed to severe grade, and pAMR3 was associated with worse cardiovascular outcomes. Desensitization strategies are mandatory to preventing the progression of AMR and treating symptomatic AMR. CAV is the most serious problem affecting allograft failure and mortality. If CAV is suspected clinically or by biomarker level, coronary angiography and/or IVUS are required for confirmation. Close monitoring is recommended consisting of regular MDCT, echocardiography, and biomarkers such as BNP, sST2, and DSA particularly anti-class II antibody. Further studies, particularly in pediatric heart transplant recipients, are necessary to understand the basic mechanisms of and develop novel therapeutic approaches to AMR, further elucidate pathogenetic mechanisms, and identify potential targets for future therapeutic interventions for CAV.
Notes
No potential conflict of interest relevant to this article was reported.