Changing prevalence of Helicobacter pylori infection in children and adolescents
Article information
Abstract
Helicobacter pylori infection has declined over recent decades. However, its prevalence remains high, and nearly 50% of the global population has been infected. In Korea, seroprevalence has steadily decreased in adults, but the status of H. pylori infection in children is unknown. The current status or trend of H. pylori infection in children is important because it can help estimate H. pylori-related diseases including gastric cancer in later life. In this review, the authors discuss the change in H. pylori infection rate among children and adolescents based on literature reviews and our research.
Key message
Although Helicobacter pylori infection rate in children is unclear due to diversity and limitation of diagnostic tests unlike in adults, investigation the childhood prevalence is important for predicting H. pylori-related diseases in the future.
H. pylori infection occurred in early childhood, and declined during 30 years in our study.
Change in risk factors of H. pylori transmission and consensus for eradication therapy in children might further reduce the infection rate.
Introduction
The prevalence of Helicobacter pylori has declined over recent decades due to improved sanitation, socioeconomic development, and better living conditions; however, its prevalence remains high in the developing world [1]. H. pylori has infected nearly 50% of the population worldwide, with rates of 35%–90% depending on population diversity and geographic area [2]. In Korea, the seroprevalence in adults has decreased over the past 13 years [3]. H. pylori infection may occur in infancy or early childhood and generally persists for life if left untreated [2,4-6]. Chronic H. pylori infection plays a pivotal role in the development of peptic ulcer, atrophic gastritis, and gastric cancer [7].
The H. pylori infection status in children versus adults is not fully known since most infected children remain asymptomatic and studies are limited. The diagnostic diversity of H. pylori can contribute to variable infection rates in children [8]. It is important to investigate the current H. pylori infection rate in children because it can be helpful for predicting the future incidence of H. pylori-related gastric diseases. This review examines the trend of H. pylori infection and risk factors associated with its transmission in children and adolescents based on a literature review and our own research.
Prevalence of H. pylori infection during childhood and adolescence in Korea
The H. pylori infection rate of symptomatic children has been steadily decreasing over the past 30 years in Korea [9-11]. Lee et al. [9] reported a decrease in the prevalence of H. pylori infection in children aged 2–15 years with recurrent abdominal pain from 25.1% to 10.8% between 1991 and 2008 in Seoul, and the infection status was determined using rapid urease tests (RUTs; commercial CLO test).
In the Kyungpook area, the H. pylori infection rate of children aged 4–16 years with recurrent abdominal pain was 7.4%; the rate did not decrease significantly from 2004 to 2014. H. pylori infection was determined by urea breath testing (UBT) in that study [11]. Jang et al. [11] found that the infection rate of children younger than 12 years was less than (6.5%) that of children aged ≥12 years (9.2%). Despite differences in investigation timing, region, and diagnostic method, the overall H. pylori infection rate appeared to decrease from 1991 to 2014 in symptomatic children in Korea [9,11].
Malaty et al. [10] reported that the infection rate in asymptomatic Korean children was 22% based on an enzyme-linked immunosorbent assay (ELISA) for anti-H. pylori immunoglobulin G (IgG) in 1996. The authors compared the infection rates of children aged 1–19 years with those of adults aged 20–75 years and found a lower prevalence in the former (22% vs. 75%).
Our research team has been investigating the prevalence of H. pylori infection since the late 1980s among asymptomatic children and adolescents using immunoblot analysis for anti-H. pylori IgG in western Gyeongnam. In this area, the H. pylori infection rate was relatively high, exceeding 80% in children older than 7 years of age among an asymptomatic population during the late 1980s [4]. Although the presence of anti-H. pylori IgG cannot be used to distinguish between past and current infection, a positive IgG result in asymptomatic children can reflect H. pylori infection status based on the fact that H. pylori infection persists unless it is treated [4,5].
The overall seroprevalence among asymptomatic pediatric patients ranging from neonates to 19-year-olds has decreased from 62.2% in 1988 to 18.2% in 2015; the age-specific infection rate has also declined. The lowest positive rate was detected in infancy and early childhood between 6 months and 4 years and has declined over the past 30 years (Fig. 1; unpublished data). Based on our investigations, the incidence of H. pylori-related diseases will decrease among adults in the future in western Gyeongnam in the absence of new infection after childhood. The infection rate in children did not differ between males and females in our investigation in concordance with previous studies [9,11].
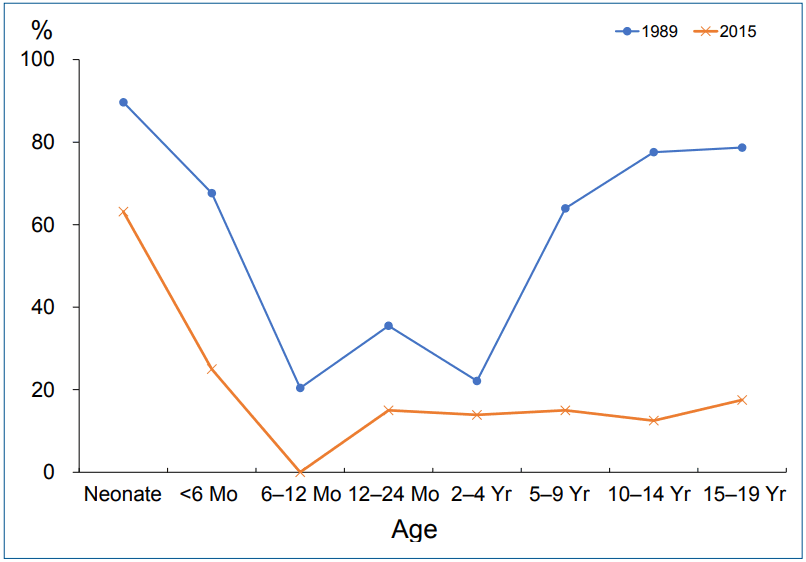
Changes in Helicobacter pylori immunoglobulin G seropositivity using immunoblot analysis among asymptomatic children and adolescents by age over the past 30 years in western Gyeongnam.
A wide range of infection rates was reported in children in previous studies, possibly caused by different living regions, diagnostic methods, ages, and symptoms of the study population [9-11].
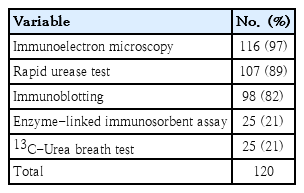
Our study using a homemade liquid-type RUT in the early 1990s detected H. pylori in 49 of 107 symptomatic children aged 2–15 years (46%). The H. pylori infection rate in symptomatic children was higher in the western Gyeongnam than in Seoul (25.1%) in the early 1990s. The difference in infection rate might be attributed to differences in the RUT used in the study and in rural versus urban areas where the study subjects resided during that time. The detection rate of H. pylori varied by diagnostic method. One of our studies suggested that immunoblot analysis was the most sensitive test among noninvasive tests for H. pylori infection in symptomatic children (Table 1; unpublished data).
The stool antigen test is a noninvasive test used to detect H. pylori infection. However, we did not investigate the diagnostic accuracy of the stool antigen test or compare it to other diagnostic methods. Epidemiologic studies in children using H. pylori stool antigen (HpSA) test are rare because HpSA has high false positive rates in children younger than 6 years and can be affected by stool patency or individual clinical condition [8,12]. RUT results also depend on the number of biopsy samples due to the patchy distribution of H. pylori in children (Table 2) [13].

Correlation between urease test results and number of biopsy samples among 255 children who underwent upper gastrointestinal endoscopy
Cross-sectional nationwide multicenter studies in an asymptomatic Korean population aged 16 years and older showed a serial decrease in seroprevalence from 66.9% to 43.9% over the past 20 years (1998–2017), with differences in the seroprevalence and decremental degree between urban and rural areas [3,14].
The H. pylori infection rate varied by age in previous studies including our research and increased with age [9-11]. Increasing seroprevalence with age might be attributed to a birth cohort effect [3]. However, new infections may also occur throughout life because anti-H. pylori IgM or IgA was positive in all age groups ranging from newborns to adults in our recent research, albeit with low rate (IgM, 5.4%–9.9%; IgA, 24.9%–30.7%; unpublished data). Seropositivities for H. pylori IgG were high in neonates and early infants in our study, which reflected seropositivity in women of childbearing age because IgG could be transmitted from mother to fetus through the placenta (Fig. 1).
Based on previous studies including our investigations, although the infection rates varied by age, diagnostic method, and region, it is clear that H. pylori infection has decreased in children and adolescents in South Korea. The declining infection rates may predict a reduced future incidence of H. pylori-related diseases in children and adults in South Korea.
Prevalence of H. pylori infection during childhood and adolescence worldwide
Although the H. pylori infection rate has decreased in South Korea and developed countries, an estimated overall 33% of asymptomatic or healthy children were still infected serologically in the world [2]. In developed countries such as Japan [15], Germany [16], the Netherlands [17], and the USA [18], relatively lower seroprevalences of 7.7%, 11.8%, 9.8%, and 5.7% were reported, respectively. However, high infection rates of 27.2%, 65.9%, 25.8%, and 40.4% were reported in Chile [19], Venezuela [20], Iran [21], and Nigeria [22], respectively.
Similar to reports of Korean epidemiologic studies, the infection rate increased with age. In cohort studies of children under 5 years of age using HpSA, the infection rate was 20% in Portugal [23] and 27.2% in Chile [19]. However, the infection rate was 42% in Portugal [23] and 77.3% in Chile [19] in studies of children older than 5 years of age using HpSA or UBT. Detection rates using UBT or HpSA in symptomatic children were 42.9% in Chile [24], 73.9% in Poland [25], 10.9% in the Netherlands [26], and 3.2% in the USA [27].
Among symptomatic children, prevalence estimates of H. pylori infection vary considerably, 3%–76% among countries and diagnostic methods, and the overall worldwide estimate was 39% [2]. The H. pylori detection rates in symptomatic children were generally slightly higher than those in asymptomatic children (around 40% vs. 33%).
Our team compared the seroprevalence of H. pylori infection among asymptomatic populations in Jinju, Korea, with those of Fukuoka, Japan. The seropositive rate of Fukuoka in the early 1990s (20%–40%) was lower than that of Jinju, Korea, in the late 1980s (50%–90%) among children and adolescents under 20 years of age. However, the infection rates in adults ≥20 years of age did not differ between the two cities. A 5-year gap existed between the subjects of the investigation [28].
A recent Japanese population-based study in which the infection rate was analyzed using HpSA and UBT in addition to serology tests showed that the prevalence of H. pylori infection in Japanese children was approximately 1.8% with no further infection occurring during the study period [29]. The infection rate was markedly decreased compared to that of our earlier report [28].
Recent epidemiologic studies showed the high prevalence of H. pylori infection. Over half of adults worldwide were infected with H. pylori [1]. H. pylori appeared to be less common in children than in adults, particularly in children of northern Europe, North America, and Japan. Conversely, it appeared to be more common in children of South America and Asia, including Korea. However, the lower prevalence among younger generations worldwide might suggest a further decline in the prevalence of H. pylori infection and associated diseases during the coming decades.
Changes in risk factors for H. pylori transmission
H. pylori transmission modes remain unclear [1]. Considering the differences in infection rates between developed and developing countries and between urban and rural areas in South Korea, socioeconomic factors appear to contribute to H. pylori transmission [1-3,9,11,28].
Due to improved economic, sanitary, and housing conditions, the overall infection rate has declined in South Korea over the past 20 years [9]. In previous studies, low income and a rural residence were the risk factors for H. pylori infection in South Korea [3,9]. An inverse association between educational level and H. pylori carrying status was also reported [30,31].
Transmission from person to person has been considered the main route [32]. According to recent Japanese studies, a significant correlation in infection status between parents and siblings suggested intrafamilial transmission of H. pylori infection. Parentto-child or grandparent-to-child transmission appeared to be an important route for the spread of H. pylori infection [15,29]. Thirty-three H. pylori isolates obtained from 11 members of three families showed familial clustering of identical random amplified polymorphic DNA fingerprinting types, which might be supportive evidence of person-to-person transmission among closely related individuals such as family members [6,33]. Many epidemiologic studies involving children showed that H. pylori infection occurred in early childhood and that the infection rate increased at around 5–7 years of age [2,28]. During these ages, children usually start to experience group life, for example, kindergarten or elementary school, which may support the possibility of person-to-person transmission.
Conclusion
Age and sex are not significantly associated with H. pylori infection in children and adults [9,11,28,34,35]. Although the infection rate increased with age in previous studies, the age-specific gradient in the prevalence of H. pylori infection appeared to be related with a birth cohort effect [3,28,34,35].
H. pylori infection may occur in early childhood and the probability of persistence is high unless it is eradicated [4,5]. A cross-sectional nationwide multicenter study in Korea showed that the H. pylori eradication therapy rate increased and the seropositive rate among adults declined inversely during the previous 18 years [14]. The low recurrence rate after successful eradication suggested that the increased rate of eradication therapy may be a minor contributing factor for the decreased prevalence of H. pylori in adults [36,37].
Despite the lack of agreement of H. pylori eradication and indications for therapy in children in Korea [38], consensus regarding the optimal anti-H. pylori management strategy may contribute to further reductions in infection rate among children.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1 A2057513). The authors declare no conflicts of interest.