Noninvasive markers for esophageal varices in children with cirrhosis
Article information
Abstract
Background
The diagnosis of esophageal varices (EV) is based on the findings of esophagogastroduodenoscopy (EGD), biopsy, and serum markers. Thus, noninvasive cost-effective tests through which high-risk EV children can be diagnosed are needed.
Purpose
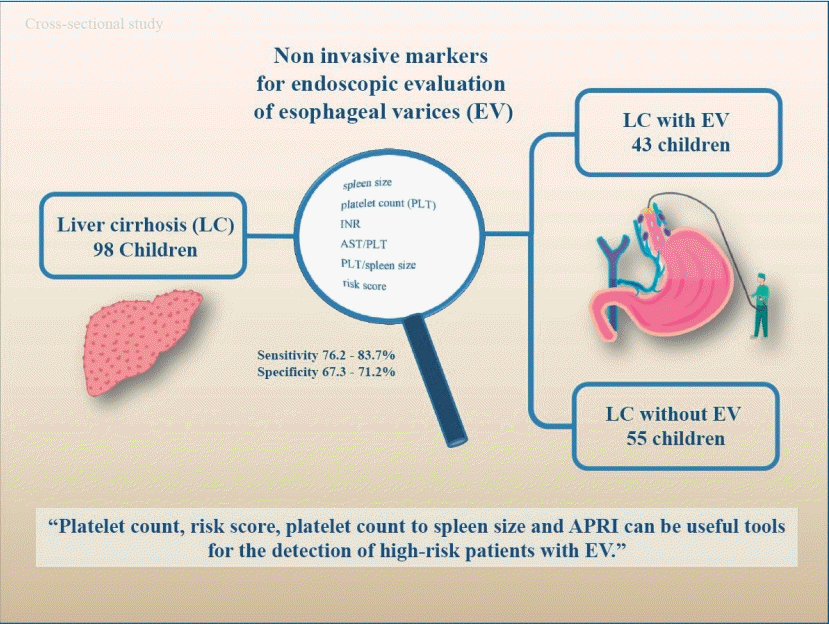
This cross-sectional study aimed to identify the noninvasive markers for EV in children with liver cirrhosis.
Methods
A total of 98 children with liver cirrhosis were evaluated in this study. The spleen size, platelet count, serum albumin, liver function test results, and risk scores were evaluated prior to endoscopy. The endoscopic investigations aimed to identify the presence of EV and red signs, and determine varices sizes.
Results
Endoscopy revealed varices in 43 subjects (43.9%). The spleen size, platelet count, international normalized ratio, aspartate aminotransferase to platelet ratio index (APRI), platelet count to spleen size ratio, and risk score differed significantly between patients with and without EV on univariate analysis; however, the logistic regression analysis showed no differences, indicating that none of these parameters were independently associated with the presence of EV.
Conclusion
Platelet count, risk score, platelet count to spleen size, and APRI can be useful tools for the identification of high-risk patients with EV and might reduce the need for invasive methods like EGD.
Key message
Question: Can noninvasive biomarkers identify esophageal varices among children with esophageal cirrhosis?
Finding: The spleen size, platelet count, international normalized ratio, aspartate aminotransferase to platelet ratio index, platelet count to spleen size ratio, and risk score differed significantly between the patients with and those without esophageal varices.
Meaning: These biological parameters can predict esophageal varices among pediatric patients and indicate the need for esophagogastroduodenoscopy.
Graphical abstract
Introduction
Liver cirrhosis (LC) is often associated with portal hypertension, which is the major cause of gastroesophageal varices, presented in 40%–85% of the cirrhosis patients [1]. Visceral bleeding is one of the serious complications associated with cirrhosis and portal vein hypertension [2], with the incidence of 20%–76% [3], therefore, early diagnosis is of great importance. Esophagogastroduodenoscopy is a gold-standard diagnostic method for varices, however, invasiveness of the technique and a significant risk associated with sedation on long-term neurological outcomes have limited its use [4]. The presence of red color and large varices mark the visceral bleeding in these patients [5]. Additionally, other imaging and labbased markers are also utilized to measure liver stiffness, platelet count, and splenomegaly are also studied, yet none of these are adequately efficient for the prediction of the risk.
There is no defined protocol for screening of EV in pediatrics, to the date. Generally, adult-based recommendations are applied in pediatric patients. Therefore, a noninvasive technique is highly demanded in children. A recent study has highlighted the use of hemostatic markers for predicting upper gastrointestinal bleeding in children with cirrhosis [6]. Moreover, end-stage liver disease models, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ratio, AST to platelet ratio index (APRI), platelet count to spleen diameter (PC/SD) have been studied as noninvasive markers for EV in several studies [7]. Nonetheless, due to controversies, clinical applications of these markers are still unknown [8].
Thus, this study is designed to investigate the role of noninvasive markers for the identification of esophageal varices in children with LC.
Methods
1. Study population
This cross-sectional study included all cirrhotic children who were referred to gastroenterology department of Children’s Medical Center, Tehran, Iran. Patients under the age of 18 years who were diagnosed with cirrhosis based on clinical, biochemical, histological (portal hypertensive gastropathy), and ultrasonographic findings were included in this study [9].
Exclusion criteria included; active hemorrhage of varicose veins at the time of referral, or history of bleeding, history of treatment for esophageal varicose or history of receiving nonselective beta-blockers or nitrates, ligation or endoscopic sclerotherapy, either surgical shunts or radiologic shunt, transjugular intrahepatic portosystemic shunt or the previous history of liver transplantation or malignancy.
2. Data collection and clinical analysis
Clinical data included age, sex, and etiology of the cirrhosis. The presence of splenomegaly, ascites, and hepatic encephalopathy were noted during the physical examination. All patients underwent esophagogastroduodenoscopy (EGD) which was performed by a single hepatologist, and variceal size and appearances (F1, F2, F3), the presence of the red signs, and variceal grading were recorded, as per the provided guidelines by the Japanese Society classification [10]. The following noninvasive markers were evaluated for the prediction of the EV: (1) platelet count using automated hematology analyzer (Sysmex XT-2000i, Kobe, Japan); (2) spleen diameter; (3) APRI test (AST/upper limit of normal/platelet count (×109/L)×100); (4) risk score: [14.2–7.1×log10 platelets (109/L)]+[4.2×log10 bilirubin (mg/dL)] (1→20) [11]; measurements were made using enzymatic method provided by PARS AZMUN (Tehran, Iran) kits by auto analyzer machine (Alcyon 300, Abbott, Abbott Park, IL, USA). Spleen diameter was assessed through ultrasonography by a single-blinded radiologist. Patients fasted 8 hours before ultrasound and patients stayed in supine position with normal breathing during the ultrasound. All tests were blinded and conducted by an experienced sonologist. The observer made 3 consecutive estimation for every variable using Aplio Color Doppler Ultrasound Unit (Toshiba, Tokyo, Japan). The doppler tracing via the lateral intercostal space with a beam angle below 20 was achieved. Portal vein and hepatic artery calculations were conducted by the software provided with the scanner.
A 2-page questionnaire comprising of demographic information, such as age, sex, date of birth, date of onset of symptoms, diagnosis history, family history, clinical and laboratory findings of patients were recorded for each patient. Laboratory examination included: platelet count, spleen size, PC/SD, AST to platelet ratio were investigated. The following data were recorded for the calculation of risk score: the underlying causes of liver disease (including biliary atresia, autoimmune hepatitis, idiopathic biliary cirrhosis, sclerosing cholangitis, cystic fibrosis), laboratory parameters such as complete blood cell, AST, ALT, international normalized ratio (INR), bilirubin, albumin, spleen size based on ultrasound, and the presence or absence of splenomegaly, and clinical signs such as encephalopathy.
Ethical approval for the study was obtained from the Tehran University of Medical Sciences Review Board (IR.TUMS. CHMC.REC.1397.037).
3. Statistical analysis
Data were presented as the mean and standard deviation, median and interquartile range, and proportions, and 95% of confidence interval (CI) were reported. A P value of <0.05 was considered statistically significant. Quantitative variables were analyzed using Student t test or the Mann-Whitney test and qualitative variables by chi-square test or Fisher exact test. With EV as the dependent variable, variables that were significant on univariate analysis, were evaluated by logistic regression model.
A receiver operator characteristic (ROC) curve was constructed and the area under the ROC curve (AUROC) was calculated. The point of highest sensitivity and specificity was determined as the cutoff value. Sensitivity, specificity, predictive values, and likelihood ratios were calculated for these cutoff values.
Results
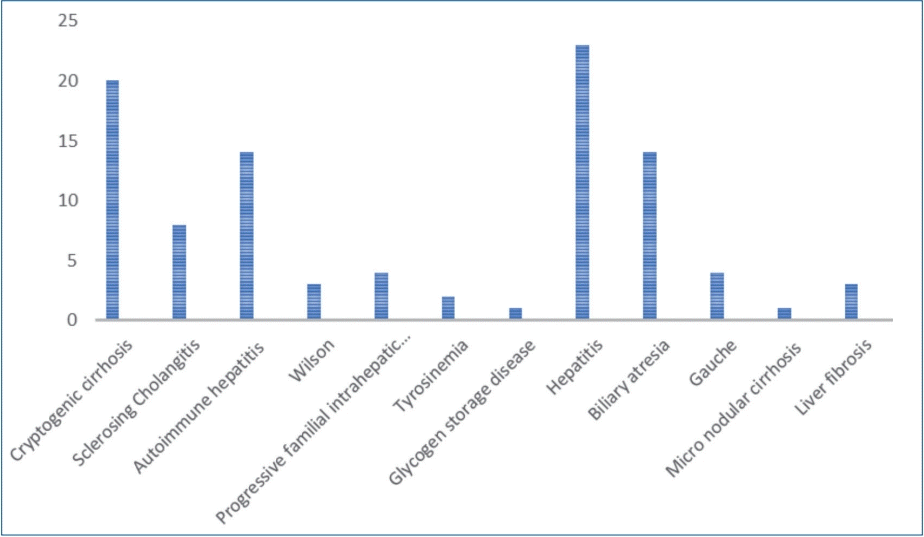
A total of 98 patients with the mean age of 9.48±4.9 years were enrolled in the study. Sixty-six patients (67.3%) were female and 32 patients (32.7%) were female. The mean body mass index (BMI) was 17.47±3.72 kg/mm2. The etiology of chronic liver disease is summarized in Table 1. Splenomegaly was seen in 95 of the patients (96.7%) and none of the patients were presented with hepatic encephalopathy. F1, F2, and F3 varices were noted in 13 (13.3%), 42 (42.9%), and 43 patients (43.9%), respectively. Varices were not detected during EGD in 55 subjects (56.1%), 43 subjects (43.9%) presented with varices. The presence of EV did not differ significantly between males and females (P=0.828). Age and etiology of the disease are not different between the groups. Spleen size, platelet count, INR, APRI, platelet count to spleen size ratio, and the risk scores were significantly different in patients with and without varices (Table 2).
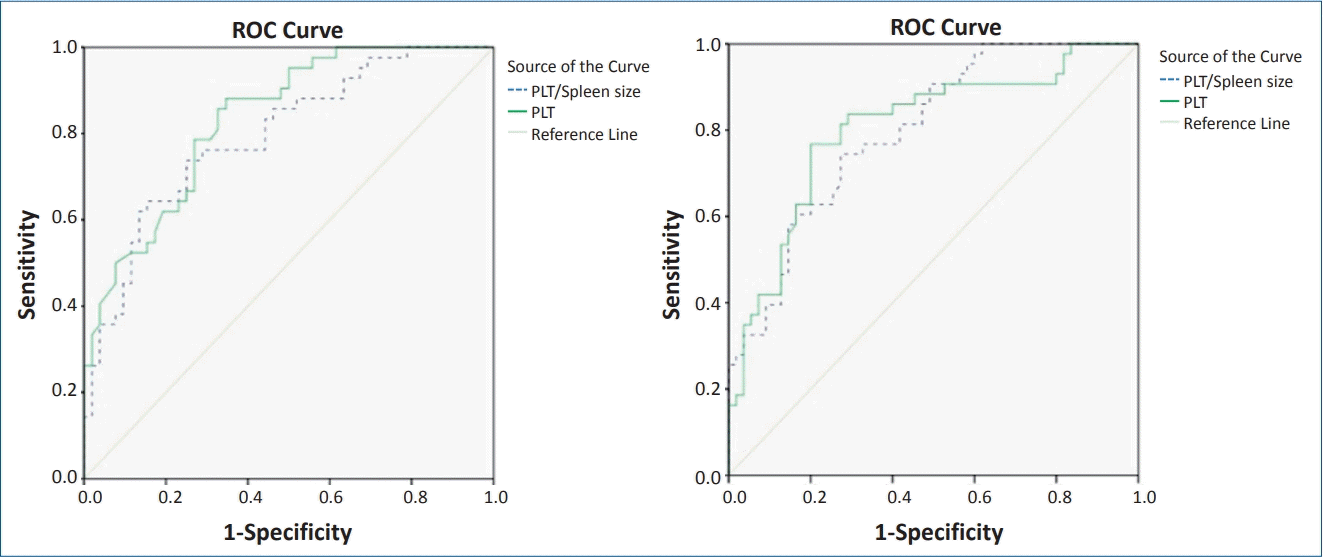
On ROC curve analysis, the best predictors of the EV included platelet count, risk score, platelet count to spleen size ratio, and APRI. The AUROC for platelet count, risk score, platelet count to spleen size ratio and APRI were 0.833, 0.804, 0.794, and 0.799, respectively (Table 3, Fig. 1). The cutoff points established with the best relationship between sensitivity and specificity for each variable are as follows: platelet count <111,000 platelets per μL (sensitivity, 83.7; specificity, 67.3), risk score >-0.82 (sensitivity, 83.7; specificity, 70.9), platelet count to spleen size <6.95 (sensitivity, 76.6; specificity, 67.3) and APRI >0.45 (sensitivity, 76.2; specificity, 71.2) (Fig. 2).
Discussion
We investigated simple, reproducible, and routinely available noninvasive parameters for esophageal varies in pediatric LC. Our study indicated platelet count <111,000 platelets per microliter, risk score >-0.82, platelet count to spleen size <6.95, and APRI >0.45 can be the significant predictors for the presence of EV. Spleen size, platelet count, INR, APRI, platelet count to spleen size ratio, and the risk score differed significantly between patients with and without EV on univariate analysis, however, the logistic regression analysis did not show any differences, which also indicates that none of these parameters were independently associated with the presence of the EV. The differences in the age, sex, and the etiology of the disease are possible indicators of these findings [12]. Several studies have been performed to identify noninvasive markers of EV in children, such as hypoalbuminemia, the Child-Pugh score, spleen diameter, thrombocytopenia, the platelet to spleen diameter ratio, Clinical Prediction Rule (CPR), and the APRI [13-15]. Other modalities, such as computed tomography scan, transient elastography, and endoscopic capsule imaging are also found useful for detecting the large EVs; though they are expensive and are not commonly available [16]. Gana et al. [17], indicated that platelet count, with a cutoff point of 115,000, is the best predictor of EV, with an AUROC curve of 0.79 (95% CI, 0.690–0.900). Our study is in line with their findings, we indicated that the platelet count with a cutoff of 111,000, can predict the EV, with an AUROC curve of 0.833 (95% CI, 0.754–0.912). In the adult population with advanced fibrosis, Park et al. [11] assessed the laboratory variables for predicting the presence of EV. They combined variables, including the platelets and bilirubin, to form a risk score. They indicated that the score had a good sensitivity and specificity with the cutoff point of -1.0. However, this study was conducted in adults. The current study found that the risk score is a good predictor of EV in children, where, the cutoff point with best sensitivity and specificity was -0.82 (sensitivity, 83.7%; specificity, 70.9%; odds ratio [OR], 12.54). According to the study performed by Adami et al. [18] in 2018 on 98 children with portal hypertension, 3 markers, including CPR (OR, 8.59), the risk score (OR, 6.09), and the platelet/spleen size z score below 25 (OR, 3.99) were reported to be good predictors of large EV. The CRP was not investigated in the current study, but our findings were in accord with their findings, in addition to risk score (OR, 12.54) and platelet/spleen size z score (OR, 7.89), we found that the APRI (OR, 6.78) and platelet count (OR, 10.57) are also good predictors. Fagundes et al. [15] evaluated 111 children in 2007 (age range, 0.7–17.6 years) and demonstrated that splenomegaly, Platelet count below the 130,000/mm3 and prehepatic and presinusoidal causes of portal hypertension could predict the presence of EV. Due to a high risk of bleeding, Molleston [19] recommended close monitoring of children with portal hypertension associated with splenomegaly and low platelets, which in association with the aforementioned factors with EV, was indicated by Fagundes et al. [15] splenomegaly via physical examination has high sensitivity and specificity for the diagnosis of EV. Being an important sign of cirrhosis and portal hypertension, splenomegaly raises the risk of EV up to 14.62 folds, which was indicated by Fagundes et al. [15]; as well as hypoalbuminemia which shows portal hypertension and a higher risk of EV (OR, 4.17). Splenomegaly was present in 96.7% of our patients, but our results did not indicate it as a predictor of EV in cirrhotic children, moreover, the mean albumin level in our study did not differ between the patients with and without EV.
Thrombocytopenia can occur in these patients as a result of several etiologies, such as immune-mediated mechanisms, lower thrombopoietin synthesis, or platelets pooled by spleen as a result of portal hypertension [20]. Without any intermediated factors associated with the pathogenesis, it has been indicated that thrombocytopenia is related directly to portal hypertension, in addition to the presence of EV [21]. Unlike adults [22], isolated platelet count could predict EV in pediatrics [23]; however, different cutoff points for platelet count have been described up to present, ranging from 100,000 mm3 to 130,000 mm3. Giannini et al. [24] suggested a platelet count to the spleen diameter ratio as a novel predictor of EV. A ratio below the 909 was indicated to be associated with EV where, the diagnostic accuracy of this parameter was 86% and the negative predictive value was 87%. In this regard, Sezer et al. [25] also demonstrated that platelet count and platelet to spleen diameter are unsuitable for detecting the EV in cirrhotic children. Adami et al. [14] reported that children with EV had lower platelet count (with cutoff 115,000) and greater spleen diameter. They also found that platelet to spleen ratio below the 1.0 discriminates patients with EV from those without EV. Although logistic regression was not statistically significant, which was explained by the authors as the differences in the age and gender, we found that it is a significant predictor of EV, with AUROC curve of 0.794, sensitivity of 76.2%, specificity of 71.2%, positive predictive value of 68.1%, and negative predictive value of 78.7%.
Kim et al. [26] suggested APRI as a good predictor of liver fibrosis. Deng et al. [27] reported that APRI value of 0.608 holds the diagnostic accuracy of EV. Our findings confirmed the accuracy of APRI as a predictor of EV in children (OR, 6.78; 95% CI, 2.74–16.75), with the sensitivity of 76.7% and specificity of 67.3%. According to Sezer et al. [25], only the presence of ascites is associated with the presence of EV in cirrhotic children (age range, 6–18 years). Nonetheless, we failed to confirm these findings in this study.
Our study has some limitations; a larger sample size should be evaluated for identifying the definite noninvasive marker of EV, which could be able to replace the routine endoscopic evaluations performed by experienced hepatologists. We did not divide our patients in age subgroups that might have provided greater specificity to our findings. Furthermore, unlike adults, liver failure in cirrhotic children derives from a variety of causes. It seems better if the accuracy of the markers is evaluated in a group of patients with the same cause of LC.
In conclusion, the findings of our study suggest that platelet count, platelet count to spleen size, and APRI can be the significant predictors for the presence of EV. Additionally, spleen size, platelet count, INR, APRI, platelet count to spleen size ratio, and the risk score differed significantly between patients with and without EV. These factors might reduce the need of invasive methods like EGD.
Notes
No potential conflict of interest relevant to this article was reported.