Association between pesticide and polychlorinated biphenyl exposure during pregnancy and autism spectrum disorder among children: a meta-analysis
Article information
Abstract
Background
The effect of exposure to environmental factors on autism spectrum disorders (ASD), especially during pregnancy, is unclear.
Purpose
This meta-analysis investigated the association between exposure to pesticides and polychlorinated biphenyls (PCBs) during pregnancy and ASD risk among children.
Methods
We searched Scopus, PubMed, Web of Science, and ProQuest for articles published through September 2019. Random-effects models were used to examine the association among studies using pooled odds ratios (ORs) and their 95% confidence intervals (CI). I2 tests were used to measure interstudy heterogeneity.
Results
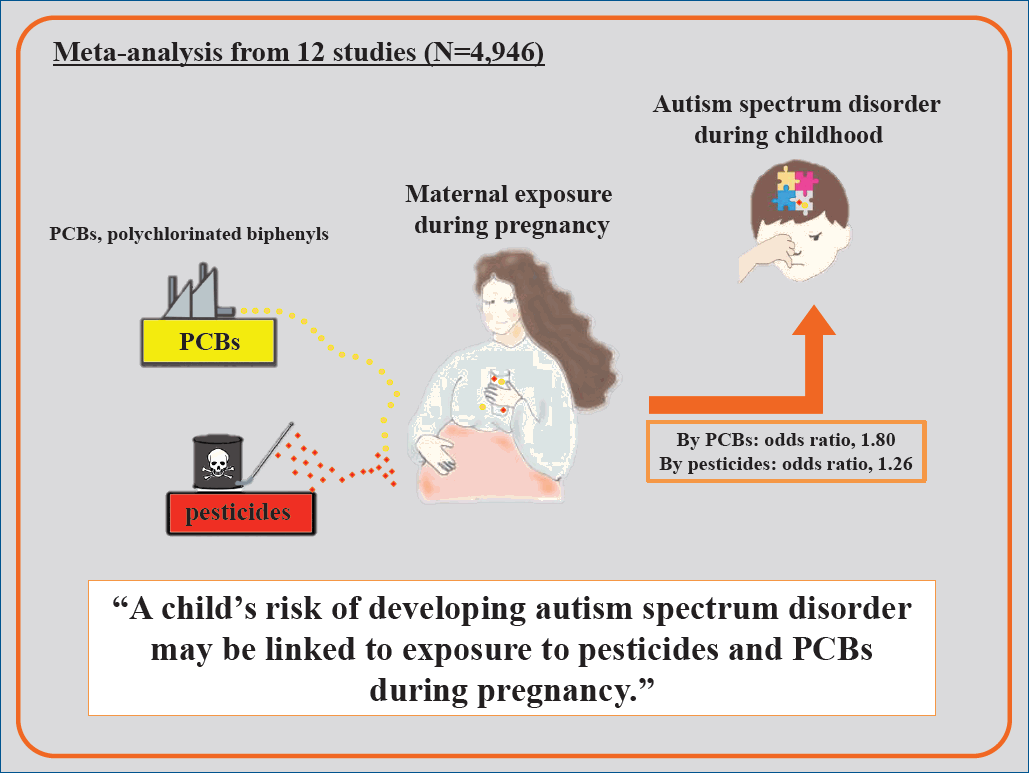
The pooled OR indicated a significant association between PCB and pesticide exposure during pregnancy and ASD risk among children (OR, 1.80; 95% CI, 1.26–2.34; and OR, 1.20; 95% CI, 1.02–1.39), respectively.
Conclusion
Findings of the present study indicate that exposure to pesticides and PCBs during pregnancy may affect the risk of ASD among children.
Key message
· This meta-analysis analyzed the association between pesticide and polychlorinated biphenyl (PCB) exposure during pregnancy and autism spectrum disorders (ASD) risk among children.
· A significant association was noted between PCB and pesticide exposure during pregnancy and ASD risk among children (odds ratio [OR], 1.80; 95% confidence interval [CI], 1.26–2.34 and OR, 1.20; 95% CI, 1.02–1.39), respectively.
· Pesticide and PCB exposure during pregnancy may affect ASD risk among children.
Graphical abstract.
Introduction
Autism spectrum disorders (ASDs) are a group of neurodevelopment disorders affecting approximately 1.5% of children in most societies [1]. The range and severity of related symptoms among children are restricted. These symptoms include repetitive patterns of behavior, difficulty with verbal and nonverbal communication, and atypical socialization [2]. An increasing pattern in the incidence of ASDs has been indicated over the last 20 years [3]. The etiology of this disease is unclear, and although many studies have reported that genetic factors play a role, they fail to provide explanation for all cases [4]. Preeclampsia, low birth weight, and neonatal icterus are risk factors for ASD [5,6]. It was recently reported that exposure to environmental chemicals during pregnancy may play an important role in the early development of ASDs [7,8].
Polychlorinated biphenyls (PCB) are a class of persistent organic pollutions in the environment with neurotoxic properties [9]. The possible mechanisms induced by PCB in the etiology of ASD include effects on neuronal development, oxidative stress, neuroexcitability, and disturbance of steroid hormone levels [10]. Many studies have investigated the impact of prenatal PCB exposure and neurodevelopmental disorders in children [11-13]. Pesticides are a class of chemical compounds, including insecticides, herbicides, fungicides, and rodenticides, that are widely used throughout the world. The toxicity mechanisms of pesticides are diverse. The changes in neuroprotein levels, gene expressions, and neurobehavioral abnormalities have been broadly reported in different studies [14]. Gunier et al. [15], Horton et al. [16], Rauh et al. [17], and Grandjean and Landrigan [18] have shown that prenatal exposure to several types of pesticides is associated with neurodevelopmental disorders and ASDs. Few studies have demonstrated no association between pesticide exposure during pregnancy and ASDs among children. Exposure to pesticides and PCBs during pregnancy is a risk factor for ASD development among children; to date, no meta-analysis has been performed on this aspect. Therefore, this is the first meta-analysis to pool all case-control and cohort studies extracted from broader databases to examine the association between pesticide and PCB exposure during pregnancy and the risk of ASDs among children.
Methods
1. Data
The major international databases (PubMed, Scopus, ProQuest, and Web of Science) were reviewed for relevant articles published through September 2019 using the following keywords: (pesticides OR DMTP OR dimethyl thiophosphate) AND (PCB OR polychlorinated biphenyls) AND (autism OR ASD OR autism spectrum disorder) with no restrictions on language or time. The reference lists of the retrieved studies were manually searched to identify additional articles.
2. Eligibility criteria
Studies were considered eligible for inclusion if they met the following criteria: cohort, case-control, or cross-sectional study design; exposure of interest was pesticides and PCB; and outcome of interest was ASD. We excluded letters to the editor, comments, reviews, and case reports.
3. Data extraction and quality assessment
Information recorded in the data sheet included the following details: first author name, year of publication, country of origin, sample size, diagnostic method, estimates (relative risk [RR], odds ratio [OR]) and their associated 95% confidence intervals (CIs), statistical adjustment for confounding factors (crude/adjusted), child’s age (mean or range), and study quality. Two independent authors (FM and EJ) assessed the studies and extracted the relevant data. In this process, any disagreements were resolved by discussion.
Study quality was assessed by checking the selection, comparability, exposure, and outcome using the Newcastle Ottawa Statement Manual (NOS) instrument [19]. According to the aforementioned items, there was a maximum of 9 devoted stars for each study. The studies with 7 or more star items were categorized as high quality, while the others were considered low-quality.
4. Statistical analysis
The OR was used to measure the association between pesticide and PCB exposure during pregnancy and ASDs among children. The combined OR and corresponding 95% CI were calculated using random-effects models. The meta-analysis was performed based on crude and adjusted form to control for confounding variables. The homogeneity of effect size was tested using the Q statistic, while the I2 statistic was used to measure interstudy heterogeneity. A funnel plot and Begg test were employed to assess possible publication bias. The data were analyzed using Stata software version 14 (StataCorp, College Station, TX, USA). Significance level was set at 0.05.
We conducted the study according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis guidelines [20].
Results
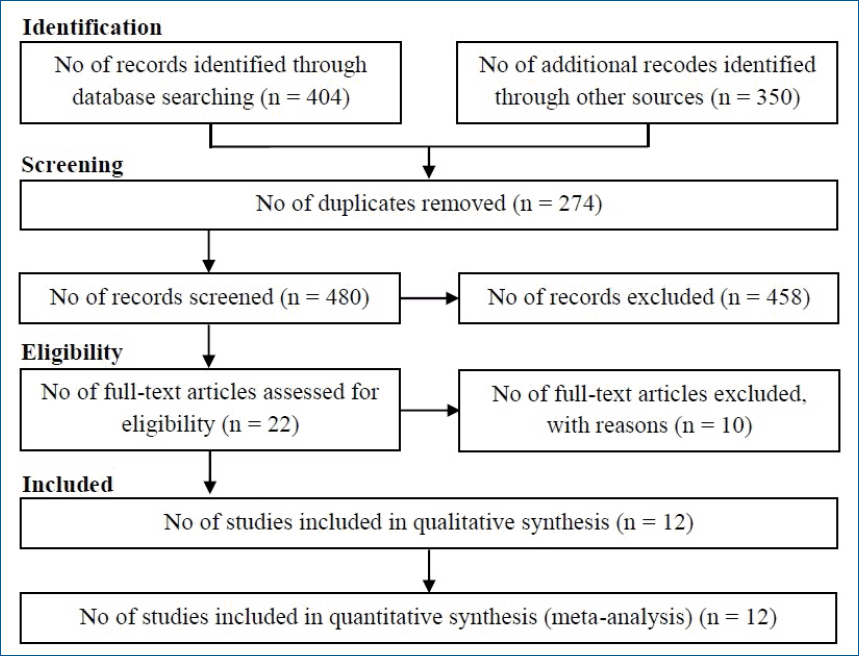
A total of 754 papers published through September 2019 were identified through advanced searches of the above-mentioned electronic databases and manual reference list searches. Of these papers, 274 were excluded for being duplicates. In the title and abstract assessment, 458 studies were excluded due to not meeting the selection criteria. In the full-text assessment, 10 were excluded. Therefore, a total of 12 studies were included in the present meta-analysis. Of them, one was cross-sectional [21], 7 were case-control [22-28], and 4 were cohort [29-32] (Fig. 1). The articles were published in English between 2007 and 2019 and included a total number of 4,946 participants.
1. Effects of exposure
Fig. 2 presents the association between PCB exposure during pregnancy and ASD among children. Based on OR estimates obtained from the studies, there was a significant correlation between PCB exposure during pregnancy and ASDs among children (OR, 1.80; 95% CI, 1.26–2.34). The results were homogenous (I2=0%, P=0.993). Table 1 shows the results of the subgroup analysis of PCB types and ASDs. Based on OR estimates obtained from the studies, there was a significant correlation between PCB138 exposure during pregnancy and ASDs among children (OR, 1.79; 95% CI, 1.14–2.44). There was no significant correlation between PCB118, PCB153, PCB170, and PCB180 and ASDs among children.

Forest plot of the association between polychlorinated biphenyls exposure during pregnancy and autism spectrum disorders among children. OR, odds ratio; CI, confidence interval.
Also, based on the OR estimates obtained from the studies, there was a statistically significant relationship between pesticide exposure during pregnancy and ASDs among children (OR, 1.20; 95% CI, 1.02–1.39). There was mild heterogeneity among the results (I2=36.0%, P=0.141) (Fig. 3).

Forest plot of the association between pesticide exposure during pregnancy and autism spectrum disorders among children. OR, odds ratio; CI, confidence interval.
Among the included studies, one study [21] reported the results based on RR. In the same study, there was a significant relationship between pesticide exposure during pregnancy and ASDs among children (OR, 1.37; 95% CI, 1.06–1.78). Cheslack-Postava et al. [22] simultaneously assessed the association between exposure to pesticides and PCB during pregnancy and ASDs among children. According to the methodology of this study, the total of 150 patients (75 subjects with autism and 75 controls) were selected and both compounds (pesticides and PCBs) were evaluated using blood sample of these patients.
2. Publication bias
Begg and Egger tests were applied to assess publication bias among studies of pesticide and PCB exposure. No publication bias among studies was detected on either test in terms of pesticide exposure (P=0.608 and P=0.503, respectively) or PCB exposure (P=0.624 and P=0.172, respectively) for ASDs among children.
Discussion
To the best of our knowledge, this systematic review and meta-analysis is the first investigation of the association between pesticide and PCB exposure during pregnancy and ASD risk among children. We evaluated the association between pesticide and PCB exposure during pregnancy and the risk of developing ASDs among different children according to available evidence from case-control and cohort epidemiological studies. The results of the present study indicate the significance of the association between pesticide and PCB exposure during pregnancy and the risk of ASDs among children.
Persistent organic pollutants are known to cause neurological diseases in humans. Once absorbed, these pollutants can remain in the body’s adipose tissue for up to 30 years [33]. PCBs are a class of persistent organic chemicals with lipophilic properties [9]. The possible mechanisms of PCBs in the etiology of ASDs include effects on neuronal development, the production of oxidative stress, neuroexcitability, and disturbed steroid hormone levels [10,11]. Kim et al. [34] and Lee et al. [35] have suggested that DNA methylation within neurons influences both single-carbon metabolism and the glutathione synthesis pathway via environmental exposure to PCBs, which alters cortical networks, long-term potentiation, and hippocampal connectivity.
Many studies have investigated the effect of prenatal exposure to PCBs and neurodevelopment disorders in children. The current study was established based on the existing literature [11,13,36]. A case-control study by Cheslack-Postava et al. [22] reported much evidence related to high PCB levels and the risk of ASD development among children aged 3–5 years. A birth cohort study by Bernardo et al. [29] showed a significant association between several PCBs and more autistic behavior. A case-control study by Lyall et al. [25] of a California population indicated clearer evidence of dose-response relationships between PCB exposure during pregnancy and the risk of ASD in the newborn. However, the effects of low-level PCB exposure on ASD remains unclear. In the current study, to assess PCB exposure during pregnancy and ASD risk among children, the association between pesticide exposure during pregnancy and ASD risk among children was evaluated. According to our findings, reports of the pooled estimate and heterogeneity regarding pesticide exposure indicated mild heterogeneity (OR, 1.20; 95% CI, 1–1.02). Therefore, there was a significant association between pesticide exposure during pregnancy and the risk of ASDs among children.
Pesticides are a class of chemical compounds that include various groups. The toxicity mechanisms of pesticides depending on the chemical structure are diverse. Insecticides are the most important group of pesticides. The main mechanism of insecticides is the inhibition of acetylcholine in the nervous system, which leads to their accumulation in the neuronal junction [37]. The changes in the signaling pathway of neurotransmitters, gene expression, abnormal thyroid hormone levels, and mitochondrial dysfunction have been widely investigated in many studies [14,38]. However, the possible mechanisms of pesticides and PCBs differ among individuals and depend on the interaction between environmental and genetic factors. Several epidemiologic studies have indicated that pesticide exposure is a potential risk factor for ASD. Based on previous studies, Sagiv et al. [39] illustrated a positive association between prenatal exposure to organophosphate pesticides and behavior like ASD among children. Considering the results obtained from many animal studies, a fetus is highly susceptible to pesticides, which can cross the placenta and affect the metabolic pathways required for their processing and excretion. Hence, prenatal exposure to pesticides can interfere with neurodevelopment [40] and motor development [38], induce oxidative stress, and lead to autismlike behavioral abnormalities [41,42]. Some researchers assessed exposure to different pesticides using maternal biological markers and reported an association between pesticides and ASD [32] or autistic behaviors [27]. Therefore, the results of previous studies and the present meta-analysis suggest that exposure to pesticides and PCBs during pregnancy may be a risk factor for ASD.
The present meta-analysis had 3 limitations. First, confounding factors were not controlled for all studies. Two studies reported a crude estimate of the confounder variables. Second, the data presented by the included studies were insufficient to allow a subgroup analysis. Third, the applied diagnostic criteria for ASD varied such that some studies did not use standardized diagnostic instruments for ASD. These limitations might have created selection bias. Despite these limitations, this meta-analysis evaluated the association between pesticide and PCB exposure during pregnancy and ASD risk among children based on epidemiological studies. Therefore, ASD risk among children may be linked to pesticide and PCB exposure during pregnancy.
In conclusion, despite the existing controversy, our findings indicate that ASD risk and development may be linked to pesticide and PCB exposure during pregnancy.
Notes
No potential conflict of interest relevant to this article was reported.