Zinc transporter 8 autoantibody in the diagnosis of type 1 diabetes in children
Article information
Abstract
Background
Type 1 diabetes (T1D) is an organ-specific autoimmune disease related to the autoimmune response against pancreatic β-cells. Zinc transporter 8 (ZnT8), an islet-specific gene product localized to the β-cell insulin granule, was recently identified as an autoantigen in T1D.
Purpose
To evaluate the use of ZnT8 autoantibody (ZnT8A) for diagnosing T1D.
Methods
This case-control study was conducted at Dr. Soetomo General Hospital, Surabaya, Indonesia, from March to May 2019. Children younger than 18 years of age with T1D based on the International Society for Pediatric and Adolescent Diabetes guideline and healthy controls were included. We measured ZnT8A level using enzyme-linked immunosorbent assay (cutoff value, 0.315).
Results
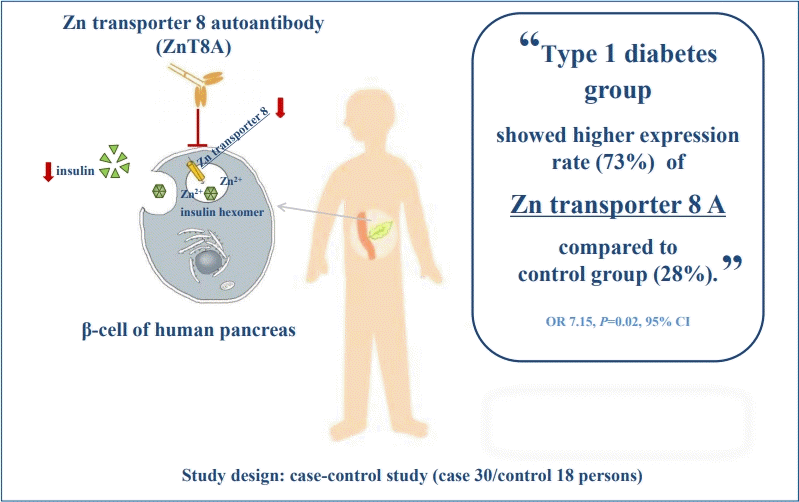
There were 30 children with T1D (50.0% boys; mean age, 11.3±3.7 years) and 18 healthy controls (44.4% boys; mean age, 8.3±3.1 years); 1 patient in each group was Madurese, while the others were Javanese. Twenty-two of 30 subjects with T1D (73.3%) tested positive for ZnT8A compared to 5 of 18 controls (27.8%) (P=0.02; odds ratio, 7.15; 95% confidence interval, 1.93–26.52). When ZnT8A-positive and -negative T1D cases were compared, no differences were detected in age at diagnosis, duration of diabetes, presence of ketoacidosis, body mass index, glycosylated hemoglobin concentration, or C-peptide concentrations.
Conclusion
ZnT8A may be useful in the diagnosis of T1D.
Key message
Question: Can zinc transporter 8 autoantibody (ZnT8A) be used for diagnosing type 1 diabetes (T1D)?
Finding: Twenty-two of 30 subjects with type 1 diabetes (73.3 %) were positive for ZnT8A compared to 5 of 18 controls (27.8%).
Meaning: ZnT8A has potential for clinical applications in the diagnosis of T1D.
Graphical Abstract. Total population (children with type 1 diabetes mellitus, T1DM) at Department of Child Health of General Hospital Dr. Soetomo were 60. Thirty children with T1DM were included into diabetes group and 18 healthy children were included into control group. We examined zinc transporter 8 autoantibody (ZnT8A) using enzyme-linked immunosorbent assay kit. We found that positivity ZnT8A in 22/30 children with T1DM (73.3%) and 5/18 in healthy children (27.8%). We conclude that diabetes group showed higher expression of ZnT8A compared to control group, so ZnT8A has potential for clinical application in the diagnosis of T1DM.
Introduction
Type 1 diabetes (T1D) is a kind of organ-specific autoimmune disease related to the autoimmune response against pancreatic β-cells. Measurement of the autoantibodies against pancreatic β-cell antigens, such as glutamic acid decarboxylase antibody (GADA), anti-insulin autoantibody (IAA), insulinoma-associated antigen-2 antibody (IA-2A), and islet cell autoantibody, is helpful to diagnose T1D. Zinc transporter 8 (ZnT8), an islet-specific gene product localized to the β-cell insulin granule, is recently identified as an autoantigen in T1D [1]. The introduction of zinc transporter 8 autoantibody (ZnT8A) in the routine diagnostic process of T1D may improve the overall autoantibody sensitivity [2]. Positivity of ZnT8A in T1D is related to the early onset of illness and increases the risk of diabetic ketoacidosis (DKA) as one ofthe complications [3]. The presence of ZnT8A in T1D was associated with ethnic that could be explained by genetic factors [4,5]. To date, study about ZnT8A as a diagnostic parameter of T1D is still limited in Asia Pacific region, especially in Indonesia. Therefore, we aimed to evaluate the use of ZnT8A for diagnosing T1D.
Methods
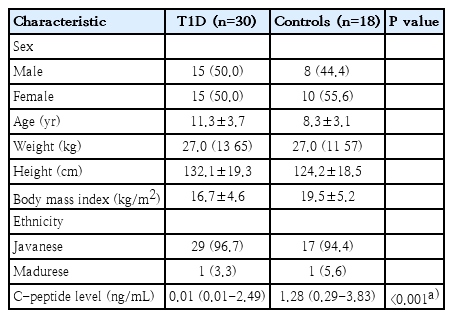
This case-control study was approved by the ethical committees of the Dr. Soetomo General Hospital, Surabaya, Indonesia (1131/KEPK/IV/2019). Written informed consent was obtained from all participants. This study was conducted in the outpatient clinic of Endocrinology Department of Dr. Soetomo General Hospital, Surabaya, Indonesia from March to May 2019. A total of 30 patients, between 1-18 years of age (15 boys and 15 girls) diagnosed with T1D according to International Society for Pediatric and Adolescent Diabetes guideline [6], were included. Eighteen healthy children with no diabetes or other autoimmune diseases were included in the study as controls.
We interviewed the subjects to collect data on their demographic, height, and weight. Body mass index (BMI, kg/m2) z score was computed. We collected the data about age at diagnosis, duration of diabetes, and history of DKA. Ketosis was diagnosed by the elevation of serum ketone bodies [7]. ZnT8A level was analyzed using enzyme-linked immunosorbent assay (ELISA) kit (Bioassay Technology Laboratory, Birmingham, UK) with the positive of cutoff value was 0.315 [8].
Data were analyzed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). After testing for normality with Kolmogorov-Smirnov test, all normally distributed data were expressed as mean± standard deviation, and nonnormally distributed data were expressed as the median (interquartile range). Categorical data were expressed with numbers or percentage. Frequency differences were compared with chi-square test or Fisher exact test when appropriate. Differences in parametric data were tested by Independent t test. Differences in nonparametric data were analyzed by the Mann-Whitney U test. P value less than 0.05 were considered statistically significant.
Results
There were 30 children with T1D (15 boys and 15 girls; aged 11.3±3.7 years) and 18 healthy children (8 boys and 10 girls; aged 8.3±3.1 years) included in this case-control study. The baseline characteristics of the patients with T1D and the controls were presented in Table 1. Twenty-two out of 30 subjects (73.3%) with T1D were positive for ZnT8A compared to 5 of 18 controls (27.8%) (P=0.02; odds ratio, 7.15; 95% CI, 1.93–26.52). The history-based, anthropometric, and metabolic characteristics of the patient with T1D were shown in Table 2. When ZnT8A-positive and -negative in T1D cases were compared, no difference was detected in age at diagnosis, duration of diabetes, presence of ketoacidosis, BMI z score, glycosylated hemoglobin concentration, and C-peptide concentrations. These variables were examined during the study.
An area under the curve (AUC) of 0.710 (95% CI, 0.553–0.867). According to ROC analysis, ZnT8A demonstrated sensitivity 73.3% and specificity 72.2% for diagnosing T1D at cutoff of 0.315 (Fig. 1).
Discussion
This study showed that ZnT8A-positive is 73.3% in Javanese children with T1D. This result is in accordance with most of the studies done in other countries. ZnT8A positivity was reported to be between 60%-80% in Caucasians (11-8 years old), 72% in Czechs (1-19 years old), 65% in Argentinians (10-32 years old), and 58.6% in Turkish children (1-18 years old) with new onset T1D [9-12]. ZnT8A positivity was reported in 24% of Chinese new onset T1D patients (1-70 years old) and differences in human leukocyte antigen (HLA) genotypes or other interethnic genetic markers were thought to be a possible cause for this low rate [13]. However, in another Asian population, Japanese acute onset T1D patients (19.1±14.5 years old) had 58% ZnT8A positivity [14]. A study from Brazil, which encompassed both a Caucasian and a non-Caucasian new onset T1D population (30.3±11.4 years old), found an overall ZnT8A positivity of 24% and it was stated that neither ZnT8A positivity nor concentration was associated with ethnicity [15]. The differences in prevalence of various types of islet autoantibodies among different ethnic populations with T1D could be explained by genetic factors especially HLA genotypes and SLC30A8 gene polymorphism [4,5]. Additionally, a recent international prospective cohort study identified a number of non-HLA genes as genetic factors for development of islet autoimmunity and T1D [16].
The ZnT8A positivity prevalence of healthy controls from different countries was reported as 1%–2.7%, which is a markedly lower rate than in this study (27.8%) [2,8,9,13]. This difference may be attributed to the larger cohorts of the other studies which better reflects the population. However, the possible effect of ethnicity cannot be excluded. ZnT8A was shown to predict risk of progression to T1D in first degree relatives of T1D patients [17]. Although these healthy controls had a negative T1D history in their families, they may have a higher risk for diabetes.
Yang et al. [13] found no difference in ZnT8A prevalence in subjects stratified with duration of diabetes. Collectively, there was no difference between the duration of type 1 diabetic patients with ZnT8A-positive and ZnT8A-negative in the present study. Another study reported that 61% of patients with T1D onset were positive for ZnT8A, and the proportion was only 33% in patients with T1D for more than 6 months [18]. However, Howson et al. [19] demonstrated a higher prevalence of ZnT8A in the first 2 years of the disease. The factors involved in the maintenance of serum ZnT8A are still to be identified, but this characteristic makes the measurement of this antibody particularly useful in patients with long-standing diabetes and unclear diabetes classification, where the identification of an autoimmune marker can establish the definite diagnosis [20].
In this study, we found the ZnT8A-positive in the T1D patient had younger age at diagnosis than with ZnT8A-negative but not significantly difference. Vaziri-Sani et al. [21] found ZnT8A titers were significantly higher in the 2- to 17-year-old type 1 diabetic patients as compared to the 15- to 34-year-old. Nevertheless, another study, which was conducted in children and adolescents who were younger than 15 years and were newly diagnosed with diabetes, reported that the ZnT8A positivity was associated with older age [3]. As above, varying ages may lead to different effects on the ZnT8A frequency. Likewise, age of onset also has an effect on the ZnT8A frequency. It was reported that the prevalence of ZnT8A declined with the increasing age at diagnosis in Chinese and Belgian patients with T1D [13,22]. Besides, the children aged 6–10 years had higher prevalence of ZnT8A than other older French within 6 months of T1D onset [18]. Taken together, it is speculated that the age at diagnosis may play an important role in the ZnT8A level.
Mbanya et al. [23] showed that the ZnT8A-positive patients with diabetes had significantly lower BMI z scores than the ZnT8A-negative ones. In the present study, the ZnT8A-positive type 1 diabetics had lower BMI z scores than the ZnT8A-negative type 1 diabetics, but not significantly.
The Diabetes Antibody Standardization Program workshop identified that ZnT8A measured by radioligand assay showed a median ROC-AUC of 0.848 [24]. Dunseath et al. [25] showed an AUC of 0.80, 54% sensitivity, and 99% specificity by conducting ROC analysis of ZnT8A by ELISA in type 1 diabetics and healthy blood donors. In this study, the ROC-AUC for ZnT8A by ELISA is 0.71, 73.3% sensitivity, and 72.2% specificity. Hence, we speculate that the ZnT8A test by ELISA may have high accuracy, not lower than the test by radioligand assay.
ZnT8A was recently identified as an autoantibody marker in T1D, which is detected earlier rather than other autoantibodies (IAA, IA-2A, and GADA) and increase diagnostic sensitivity of T1D [26]. The study is limited by a relatively small number of subjects. The conclusion of this study is ZnT8A has potential for clinical applications in the diagnosis of T1D.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The authors wish to thank the patients who participated in the study and the endocrine teams of Dr. Soetomo Hospital, Surabaya, Indonesia, for the support.