Extracorporeal membrane oxygenation with systemic heparinization as a rescue therapy for acute life-threatening pulmonary thromboembolism complicating nephrotic syndrome
Article information
Key message
Question: How would you treat acute life-threatening pulmonary thromboembolism occurring in nephrotic patients?
Finding: A 16-year-old woman with minimal change-associated nephrotic syndrome presented with cardiac arrest caused by an acute bilateral pulmonary thromboembolism. Her hemodynamics stabilized with resolution of thrombi while on venoarterial extracorporeal membrane oxygenation (ECMO) and systemic heparinization.
Meaning: In selected cases, ECMO with systemic heparinization may rescue patients from acute life-threatening pulmonary thromboembolism even without reperfusion therapy.
A 16-year-old female was admitted with a 2-week history of generalized edema and weight gain of 10 kg. The initial laboratory findings were as follow; hemoglobin, 15.4 g/dL; platelet, 304×103/μL; blood urea nitrogen, 12 mg/dL; creatinine, 0.59 mg/dL; protein, 4.7 g/dL; albumin, 1.8 g/dL; total cholesterol, 459 mg/dL; fibrinogen, 642 mg/dL; C3, 156.0 mg/dL; C4, 30.1 mg/dL; CH50, 42.4 U/mL; ANA, negative; anti-dsDNA, 4.79 IU/mL. Urinalysis showed 4+ protein and trace blood, and spot urine protein/creatinine was 14.2 g/g. A kidney biopsy revealed minimal change disease, and she received prednisolone 60 mg daily (1 mg/kg/day) for 10 days and intravenous albumin and furosemide as needed. Thereafter, she was discharged with prednisolone (60 mg/day). Five days after discharge, she suddenly developed upper back pain and shortness of breath.
On arrival at the emergency room, she became cyanotic with decreased consciousness. Blood pressure (BP) was 90/60 mmHg and heart rate was 130/min. Arterial blood gas analysis showed severe hypoxemia with pH, 7.30; pCO2, 36 mmHg; pO2, 46 mmHg; HCO3−, 17 mmol/L; and O2, saturation 76% while she was on ambu-bagging with oxygen administration. After 30 minutes, BP was not measurable with no palpable pulse, and cardiopulmonary resuscitation (CPR) was performed immediately. Following 1 minute of CPR, BP was measurable. However, a second and a third cardiac arrest occurred every 5 minutes, and the oxygen saturation did not reach above 80% despite the mechanical ventilation with fraction of inspired oxygen (FiO2) 1.0.
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) was immediately applied to the right femoral vein and artery (blood flow, 4.45 L/min; FiO2, 1.0; gas flow, 4.5 L/min). Echocardiogram revealed an enlarged hypokinetic right ventricle, an increased right ventricular systolic pressure and a D-shaped left ventricle, suggesting pulmonary embolism. In a chest computed tomography (CT) angiography, multiple thromboemboli were found in bilateral main pulmonary arteries and their branches (Fig. 1A, B).
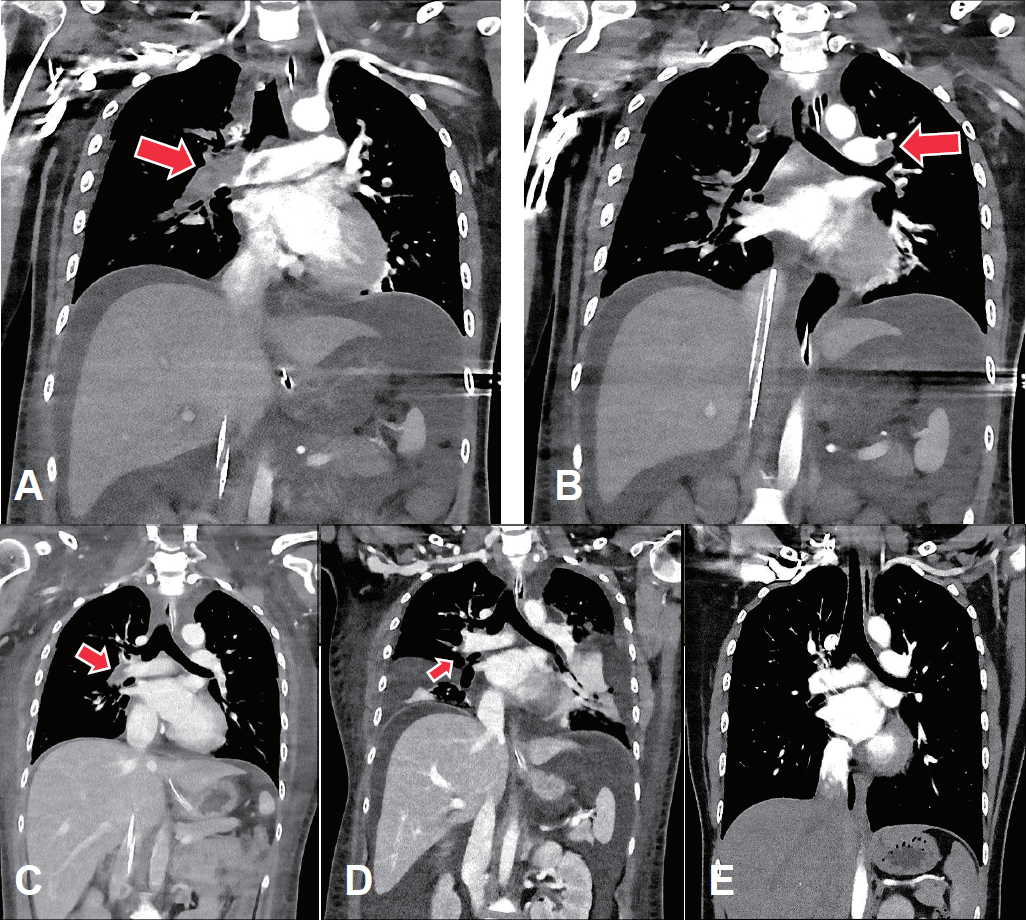
The computed tomography (CT) scan findings of the pulmonary thromboembolism. The initial CT scan shows near-complete obstruction of the right main pulmonary artery (A) and partial obstruction of the left main pulmonary artery (B) with thrombi (arrows). (C) On the 8th day of extracorporeal membrane oxygenation, the extent of the thrombi decreased, especially in the right main pulmonary artery. (D) Thereafter, the thrombi decreased further; however, the degrees of bilateral pleural effusion and ascites increased. (E) At 2 months after discharge, there was no residual thromboembolism in either main pulmonary arteries or their branches.
While ECMO was maintained, heparin was initiated and adjusted to keep activated partial thromboplastin time at 60–65 seconds. For nephrotic syndrome, methylprednisolone (60 mg/day), albumin and furosemide were administrated intravenously. On the sixth day of readmission, she became hemodynamically stable with a marked improvement of right ventricular function on echocardiography, and ECMO was removed. Heparin was switched to enoxaparin and then to warfarin. On the eighth day of readmission, CT scan showed a decrease in the extent of pulmonary embolism (Fig. 1C). On the tenth day of readmission, antithrombin III level, protein C activity, and protein S activity were measured and were within normal limits, and continuous renal replacement therapy was initiated to control volume overload complicating nephrotic syndrome and acute kidney injury (serum creatinine, 1.38 mg/dL).
However, generalized edema and bilateral pleural effusion persisted with proteinuria greater than 8 g/day (Fig. 1D). Thus, cyclosporine (200 mg/day) was added to the steroid, and eventually urinary protein decreased below 1 g/day and generalized edema improved. She was discharged without complications, and a follow-up chest CT revealed the complete resolution of pulmonary thromboembolism (Fig. 1E). The medications were then tapered off (Fig. 2), and she experienced no relapse with serum creatinine 0.65 mg/dL and spot urine protein/creatinine 0.1 g/g at 3 years after discharge.

Treatment modalities and the change in the level of proteinuria as measured by spot urine protein/creatinine ratio. ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy.
Q: Which one was reported as a risk factor for thromboembolism in nephrotic syndrome?
1) Low serum albumin
2) High level of antithrombin III
3) Low level of fibrinogen
4) High level of protein S
In nephrotic syndrome, thromboembolism was reported in approximately 24% of adults and 2.8% of children, and manifests as deep venous thrombosis, renal vein thrombosis, or pulmonary embolism [1]. Thromboembolic risk increases with the severity of hypoalbuminemia [2,3], and prophylactic anticoagulation is considered at very low serum albumin [3]. The hypercoagulability is caused by urinary losses of antithrombotic factors such as antithrombin III, protein C, protein S, and increased hepatic synthesis of pro-thrombotic factors such as factors V, VII, VIII, X, von Willebrand factor, and fibrinogen [3].
Treatment modalities of acute pulmonary thromboembolism depend on hemodynamic stability. Patients without hemodynamic compromise are treated with anticoagulation alone [4]. However, reperfusion therapy (systemic thrombolysis, percutaneous catheter-directed thromboaspiration/embolectomy, or surgical embolectomy) is recommended if persistent hypotension, obstructive shock or cardiac arrest occurs [4]. In refractory circulatory collapse or cardiac arrest, ECMO may also be needed to maintain circulation and tissue oxygenation [4].
So far, there have been several case reports of fatal or life-threatening pulmonary thromboembolism associated with nephrotic syndrome [5-8], and ECMO was used in one case who underwent surgical embolectomy and then was placed on VA-ECMO for right ventricular failure [8]. In contrast, our patient did not undergo reperfusion therapies but became hemodynamically stable with decreases in the extent of thrombi while on ECMO. It may be because the thrombi were dissolved by endogenous fibrinolysis while heparin prevented further thrombus formation [9].
Like our case, patients undergoing ECMO due to acute massive pulmonary thromboembolism may recover with anticoagulation alone [9,10]. However, surgical embolectomy is necessary if there is no improvement of right ventricular function [4,10]. The chronicity of the thrombus was suggested as a factor necessitating early surgical intervention [10].
Answer: 1
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was approved by Institutional Review Board of Dankook University Hospital (DKUH 2020-04-005).
