Pediatric postintensive care syndrome: high burden and a gap in evaluation tools for limited-resource settings
Article information
Abstract
This article aimed to summarize the impact and burden of pediatric postintensive care syndrome (PICS-p) in the physical, mental, cognitive, and social health domains after a review of the current pediatric literature in MEDLINE and PubMed. We also aimed to elucidate the limitations of the current evaluation tools used in limited-resource settings. PICS-p can impact a child’s life for decades. Most validated tools are time-consuming, require qualifications, and expertise, are often limited to older children, and can evaluate only one domain. A novel, simple, and comprehensive surveillance tool can aid healthcare providers in the early detection and intervention of PICS-p. Further studies should validate and refine the parameters that will enhance the outcomes of pediatric intensive care unit survivors.
Key message
Pediatric postintensive care syndrome has high impact and burden and can affect a child’s life for decades. The early evaluation and detection of such problems require a simple and less time-consuming surveillance tool. Current evaluation tools can be difficult and strenuous for areas with limited resources. Thus, a new simple tool is required for the early detection and intervention of postintensive care syndrome in critically ill children.
Graphical abstract
Introduction
Millions of children worldwide experience critical illness that requires a pediatric intensive care unit (PICU) admission. With medical and technological advancements, mortality rates have decreased substantially. Nevertheless, in both adult and pediatric critical care, decreased mortality is often accompanied by increased morbidity [1-3]. Thus, mortality rate alone is no longer a measurement of success in patient care. In the 2010 Society of Critical Care Medicine Conference, a novel concept of postintensive care syndrome (PICS) was proposed and defined as “new or worsening impairments in physical, cognitive, or mental health status arising after critical illness and persisting beyond acute care hospitalization.” Children admitted to the PICU were also classified as being at high risk of developing PICS [3,4].
Since that conference and a publication by Needham et al. [3] in 2012, significant attention has been directed toward detection and early interventions to improve PICS-related outcomes [4]. Although PICS is well defined in the adult literature, data in pediatrics remain limited. Data concerning pediatric PICS (PICS-p) started to emerge after the systematic review by Herrup et al. [5] in 2017 and Watson et al. in 2018 [6]. Compared to adulthood, childhood is dynamic with heterogeneity in age, developmental milestones, social status, cognitive development, and physiologic capacities. Furthermore, children are also dependent on their family and caregivers. Thus, a conceptualizing framework concerning PICS-p was categorized by Manning et al. [1] in 2018 into physical, cognitive, mental, and social health domains. Many validated tools such as the Chalder Fatigue Scale, Health Utility Index (HUI), Strength and Difficulties Questionnaire, or Child-revised Impact of Event Scale were used in an attempt to detect PICS in children in different domains. A model was developed and utilized at St. Louis Children’s Hospital and Doernbecher Children’s Hospital to assess anxiety, depression, posttraumatic stress disorder (PTSD), and delirium in children admitted to the neurological critical care unit as well as their parents. This model consisted of several tools and checklists for use at baseline, PICU discharge, and hospital discharge as well as during outpatient visits [7]. As evidence of PICS-p started accumulating in the literature, it is of the utmost importance to explore the burden of such problems as well as the limitations of the current evaluation tools, especially in limited-resource settings. The main aim of this article was to summarize the impact and burden of PICS-p from the current pediatric literature in all 4 domains. The secondary aim was to discuss the problems with the current PICS-p evaluation tools.
Methods
This review article followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Strategy for the selection of relevant articles (Fig. 1). The population, intervention, comparator, and outcome for this review were as follows: population—children aged 1 month to 18 years who were admitted to the PICU regardless of conditions and were evaluated for PICS-p, including family members caring for critically ill children; intervention/comparator—different PICS-p evaluation tools; and outcome—the burden of PICS-p in all 4 domains using different evaluation tools.
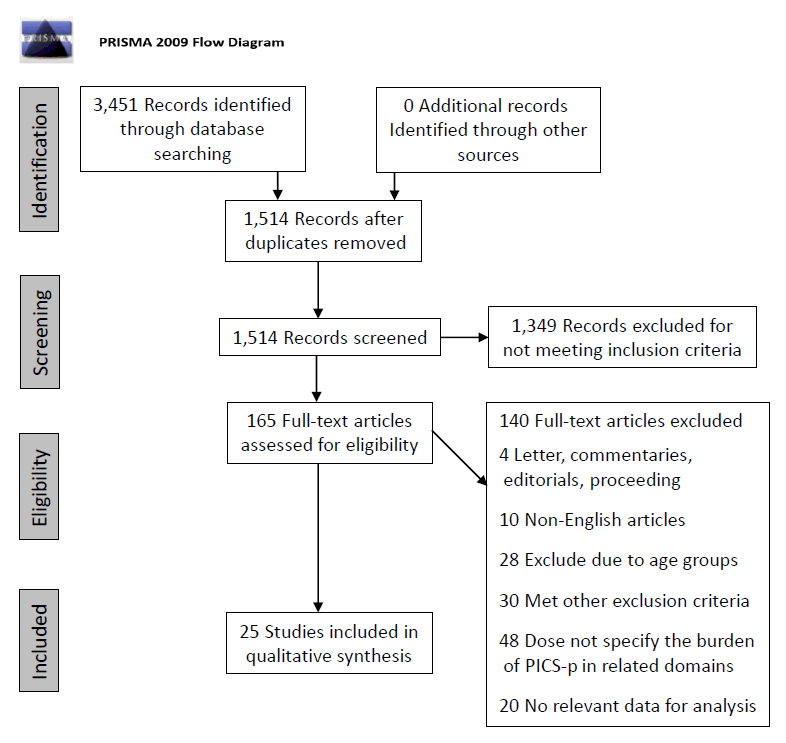
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram. PICS, postintensive care syndrome.
All retrospective, prospective, and cross-sectional studies evaluating the burden and morbidities of physical, cognitive, mental, and social health of the children surviving PICU stays as well as their families were included. Studies focusing on specific diseases, such as those of children admitted with neurologic diseases and congenital heart diseases, were also included for further elucidation of the burden of PICS-p in children. Due to the neurodevelopmental patterns and physiological differences from the PICU subpopulation, studies including neonates admitted to the neonatal intensive care unit were excluded.
A literature search was conducted of MEDLINE and PubMed in July 2020 using keywords and search terms. Two reviewers (CC and PT) were initially responsible for the eligibility evaluation. The reviews were performed independently. Agreement on the inclusion and exclusion of the studies was further discussed with the third reviewer (RO) in cases of disagreement. The search strategy and search terms are described in Supplementary material 1. Articles were included from database inception to the search timing. Only English-language articles were included in the analysis. Duplicate studies were excluded; letters, commentaries, editorials, guidelines, proceedings, conference abstracts, and case reports were also excluded from the analysis. Additionally, studies that did not specify definite age groups; included unclear populations or subgroups; or included adults without a subgroup analysis of pediatrics or unrelated to the family of critically ill children were excluded. Lastly, if the evaluation tools were not suitable for the age groups, such as those designed for adults or not fully validated, the articles were excluded from the analysis.
To fulfill the secondary study objective, qualitatively analyzed studies were further evaluated in terms of the tools used to quantify and evaluate PICS-p to identify possible limitations of current evaluation tools.
Results
After the removal of duplicates, a total of 1,514 search results were obtained. The abstracts were screened for relevance. At the end of the screening, approximately 165 articles were subjected to full-text review. Review articles were also evaluated for relevant data. After careful evaluation, 140 articles met the exclusion criteria and were excluded (Fig. 1). Thus, a total of 25 articles were qualitatively reviewed. The results are summarized by domain below.
1. Physical and functional impairment
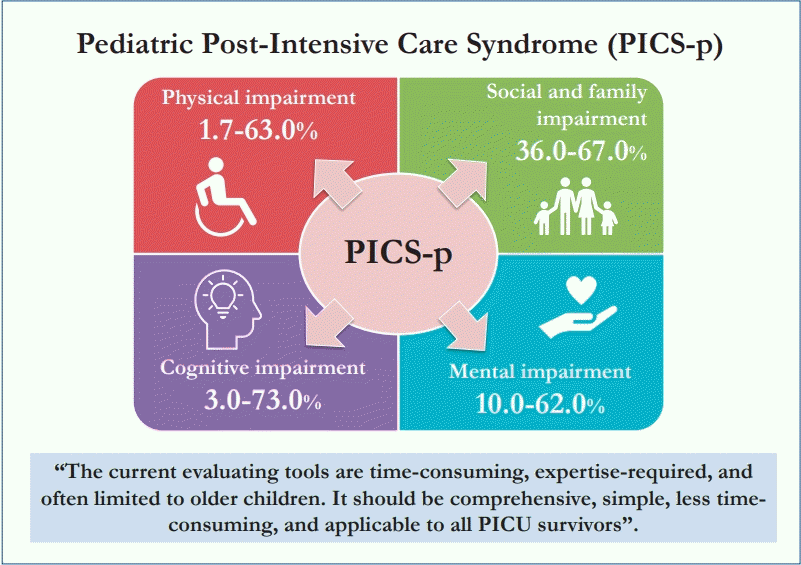
Two systematic reviews, 5 prospective cohort studies, 1 descriptive study, and 1 database review were analyzed for physical and functional impairments. Studies demonstrated that physical impairments were 1.7%–63% in PICU survivors among different age groups using different evaluation tools. The impact of intensive care-acquired weakness and functional impairments is summarized in Table 1 [8-14].
One systematic review and 1 pilot study were evaluated for sleep problems in PICU survivors [15,16]. A systematic review by Kudchadkar et al. [15] presented 9 cross-sectional studies concerning sleep in critically ill children, which demonstrated sleep fragmentation, increased stage 1 and stage 2 sleep, and decreased rapid eye movement sleep. Within this systematic review, a study by Gottschlich et al. [17] demonstrated an absence of stage 3 and 4 sleep in 40% of polysomnographic periods with improvements upon recovery in PICU patients admitted with burns. Polysomnographic studies in mechanically ventilated children under neuromuscular blockade also showed sleep fragmentation and sleep stage distribution variations as well as significant circadian disruption [16].
Different studies used different evaluation tools to quantify physical impairments. Banwell et al. [9] used neuromuscular examination, Als et al. [8] utilized the Chalder Fatigue Scale for physical evaluation, and 3 other studies utilized the HUI System [11-13]. The tools currently used to evaluate the physical and functional domain have certain problems. The Chalder Fatigue Scale, an 11-item Likert fatigue scale, measures the extent and severity of fatigue and differentiated tiredness from chronic fatigue syndrome [18,19]. Since this scale is a self-rating Likert scale, it is rather limited to older children and cannot detect problems in younger individuals. The HUI-2 and HUI-3 surveys evaluate 15 health attributes of 3–6 levels of abilities/disabilities. The HUI surveys can comprehensively assess patients with unique health statuses and provide an extensive framework to describe health status and health-related quality of life [20]. Nevertheless, the HUI has 2 major drawbacks. First, it is scored using single- or multiple-attribute utility functions, which include look-up tables and mathematical formulas, making its use difficult in a busy setting. Second, use of the health status classification system based on the HUI is not suitable for children younger than 5 years of age.
2. Cognitive health impairment
A prospective observational study by Als et al. [21] revealed that PICU survivors scored lower on measures of verbal and visual recall, visual sustained attention, and working memory capacity, particularly the septic and neurologic disease cohort. Widespread cognitive impairments were found in younger children with lower socioeconomic status. A follow-up study demonstrated persistent impairments at 12 months despite improvements [22]. Studies using the Pediatric Cerebral Performance Category revealed new cognitive problems in 3%–73% of survivors [23]. Among 29,352 PICU admissions from 24 virtual PICU sites, 3.4% of children demonstrated cognitive decline [24].
The Children Memory Scale used by Als et al. [21,22] was designed to comprehensively assess learning and memory in children and adolescents aged 5–16 years. The average time to complete this complete battery of tests was 30–40 minutes [25]. Similar problems were also applied to the Weschler Abbreviated Scale of Intelligence used in a similar study. Despite its ability to assess specific and overall cognitive capabilities, its use is limited to children older than 6 years. It consists of 4 battery of subtests: vocabulary (31 items), block design (13 items), similarities (24 items), and matrix reasoning (30 items) [26]. The use of these batteries might not be suitable for low-capacity areas. Furthermore, these tests are classified as level C measures, meaning that they must be administered and interpreted by individuals with a doctorate degree in psychology or related discipline. Examiners with a bachelor’s degree in a related discipline might be able to conduct the test under supervision [25,26].
3. Mental health impairment
Children can experience mental health issues such as depression, anxiety, or even PTSD. Five prospective cohorts and 1 retrospective cohort revealed a substantial amount of mental impairment in critically ill children [27-32]. A prospective cohort study by Judge et al. [30] illustrated that as high as 62% of PICU survivors experienced PTSD symptoms and 10% were diagnosed with probable PTSD. Another prospective study by Shears et al. [32] showed that 15% of critically ill children with meningococcal disease had PTSD. The data concerning the impact of mental impairment are summarized in Table 2 [27-32].
4. Social health and family
Studies have shown that as high as 67% of family members suffered from depression during the first week of PICU admission and as high as 49% experienced PTSD symptoms at 6 months. Approximately 36% of caretakers reported burden or overload within 2 months of the PICU admission [30,33-35]. A study by Shears et al. [32] reported that 38% of mothers and 19% of fathers were at risk of developing PTSD as evidenced by higher maternal Impact of Events Scale Scores in the PICU.
The Impact of Event Scale, Strength and Difficulties Questionnaire, and Child Behavior Checklist are often used to evaluate the behavioral and psychological functions of children after critical illness [7,28,30-32]. These evaluation tools were mostly self- or parent-reported, were often restricted to older children, and can be time-consuming [36-38].
The evaluation tools and their disadvantages are described in Table 3.
Discussion
Our study revealed the high impact and burden of PICS-p in critically ill children and demonstrated several problems with the current PICS-p evaluation tools.
As shown in the results within the physical and functional impairment domains, critically ill children are at high risk of developing intensive care-acquired weakness and sleep disruption. Intensive care-acquired weakness is described as acute symmetric muscle weakness in the extremities caused by critical illness. It can be classified as critical illness polyneuropathy, critical illness myopathy, critical illness neuromyopathy, and muscle deconditioning [33,39]. The etiologies are usually multifactorial but predominantly associated with excitation-contraction uncoupling, altered muscle biogenetics, and altered membrane excitability [40]. Its major risk factors include sepsis, prolonged mechanical ventilation, immobilization, glucocorticoids, and neuromuscular blocking agents [33]. Critically ill children are also exposed to multiple environmental, pharmacological, and physical factors that might lead to sleep disruption and poor sleep quality. Exposure to narcotics and sedatives decreases slow-wave sleep and rapid eye movement sleep. A systematic review of sleep in critically ill children by Kudchadkar et al. [15] demonstrated that critically ill children had sleep fragmentation and circadian disruption. Most studies utilized polysomnography to assess sleep quality and problems. This can be cumbersome in areas with limited availability of polysomnography and sleep technicians.
This article also demonstrated that critically ill children suffered from cognitive impairment and mental health dysfunction after intensive care unit discharge. Cognitive impairment can include deteriorations in memory, executive function, language, and visuospatial abilities [33]. Poor glycemic control, an admission diagnosis of trauma, injury and poisoning, delirium, and in-hospital acute stress symptoms are risk factors for cognitive decline [7,33,41]. Children suffering from septic illness, experiencing higher numbers of invasive procedures and interventions, or receiving higher dosages of benzodiazepines and narcotics are at higher risk of experiencing mental health deterioration [5,33,42].
Not only did children experience functional deterioration, family caring for PICU survivors also suffered. The impact on social health may include problems going back to school and getting along with peers and siblings, and caring for a sick child can have negative psychological effects [5,31].
In this review, the burden of PICS-p was elucidated in different domains via different prospective studies and review articles. Nevertheless, there were certain limitations to this review. There were vast differences in definitions, measurement tools, and follow-up timings among studies. Heterogeneity among study protocols might have led to underestimation and overestimation of the impact of PICS-p since some impairments might not be evident during the early phase of PICU discharge. These differences also posed problems in terms of when to assess which tests should be used, and how long these children should be followed up. As mentioned above, children demonstrate a diverse functional status related to age and developmental stage, and it is rather difficult to establish a preadmission baseline to demonstrate the full impact of PICS-p. As summarized in Table 3, most currently validated tools in the literature are time-consuming, require qualifications and expertise, are often applicable only to older children, and can evaluate only one domain. Children’s cooperation also plays a role in the evaluation, making it difficult to obtain accurate results in a timely fashion. The Pediatric Cerebral Performance Score and Pediatric Overall Performance Category are 2 simple validated tests that were not time-consuming and did not require a high level of expertise. Nevertheless, these 2 tests are only concerned with functional and cognitive morbidity and are not fully comprehensive. A simple, cheap, less time-consuming, and comprehensive surveillance tool that considers every domain and can be performed by everyone and in every child surviving a PICU stay is required for use in limited-resource settings.
In conclusion, PICS-p can impact a child’s life for decades. Current evidence and evaluation tools have use limitations in limited-resource settings. Thus, a simple and novel surveillance tool is needed to aid healthcare providers in the early detection of and intervention for PICS-p.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The author would like to show appreciation to the Department of Pediatrics, Faculty of Medicine, Thammasat University Hospital for the strong support and extensive cooperation in making this study possible. The author would also like to especially show a great appreciation to Professor Paskorn Sritipsukho, Department of Epidemiology, Faculty of Medicine, Thammasat University for the great advice in the improvement of this manuscript.
Supplementary material
Supplementary material 1 can be found via http://doi.org/10.3345/cep.2020.01354.
Search strategy for systematic review