Clinical features, diagnosis, and outcomes of multisystem inflammatory syndrome in children associated with coronavirus disease 2019
Article information
Abstract
The novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been spreading worldwide since December 2019. Hundreds of cases of children and adolescents with Kawasaki disease (KD)–like hyperinflammatory illness have been reported in Europe and the United States during the peak of the COVID-19 pandemic with or without shock and cardiac dysfunction. These patients tested positive for the polymerase chain reaction or antibody test for SARS-CoV-2 or had a history of recent exposure to COVID-19. Clinicians managing such patients coined new terms for this new illness, such as COVID-19–associated hyperinflammatory response syndrome, pediatric inflammatory multisystem syndrome temporally associated with COVID-19, or COVID-19–associated multisystem inflammatory syndrome in children (MIS-C). The pathogenesis of MIS-C is unclear; however, it appears similar to that of cytokine storm syndrome. MIS-C shows clinical features similar to KD, but differences between them exist with respect to age, sex, and racial distributions and proportions of patients with shock or cardiac dysfunction. Recommended treatments for MIS-C include intravenous immunoglobulin, corticosteroids, and inotropic or vasopressor support. For refractory patients, monoclonal antibody to interleukin-6 receptor (tocilizumab), interleukin-1 receptor antagonist (anakinra), or monoclonal antibody to tumor necrosis factor (infliximab) may be recommended. Patients with coronary aneurysms require aspirin or anticoagulant therapy. The prognosis of MIS-C seemed favorable without sequelae in most patients despite a reported mortality rate of approximately 1.5%.
Key message
Hundreds of cases of children and adolescents with hyperinflammatory responses such as Kawasaki disease have been reported amid the coronavirus disease 2019 (COVID-19) pandemic, leading to coining of the new term COVID-19–associated multisystem inflammatory syndrome in children. In this review article, we introduce the illness and describe its case definitions, epidemiology, pathogenesis, clinical features, treatments, and outcomes.
Introduction
The new coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been spreading worldwide since December 2019, resulting in enormous numbers of affected patients and substantial mortality in many countries. The proportion of children affected by COVID-19 was small compared to adults, and most affected children were asymptomatic or presented with mild symptoms [1-3]. However, some children and adolescents with Kawasaki disease (KD)-like hyperinflammatory illness were reported in Europe and the United States (US) during the peak of the COVID-19 pandemic in the spring of 2020 [4-7]. Hundreds of such cases have been reported since April 2020, and a few died of the illness [4,7]. They presented with fever, skin rash, conjunctivitis, oral mucosa changes (red fissured lips, strawberry tongue), and hand or foot edema, all of which are included in the diagnostic criteria of KD, in addition to prominent gastrointestinal symptoms (abdominal pain, vomiting, diarrhea). They also had markedly elevated inflammatory markers (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP], procalcitonin, ferritin, interleukins [ILs], etc.), and neutrophilia. Some had cardiac abnormalities (left ventricular dysfunction, myocarditis, pericarditis, valvular regurgitation, coronary arterial ectasia, or aneurysm) or symptoms and signs of shock (hypotension, hypoxemia, altered consciousness). The patients tested positive for the reverse transcription-polymerase chain reaction (RT-PCR) or antibody test for COVID-19 or had a history of recent contact with COVID-19 patients. Clinicians managing such patients coined new terms for this new illness, COVID-19–associated hyperinflammatory response syndrome, pediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS), or COVID-19–associated multisystem inflammatory syndrome in children (MIS-C). In this article, we use the term MIS-C and review its case definitions, epidemiology, pathogenesis, clinical features, treatments, and outcomes.
Case definition and diagnosis of MIS-C
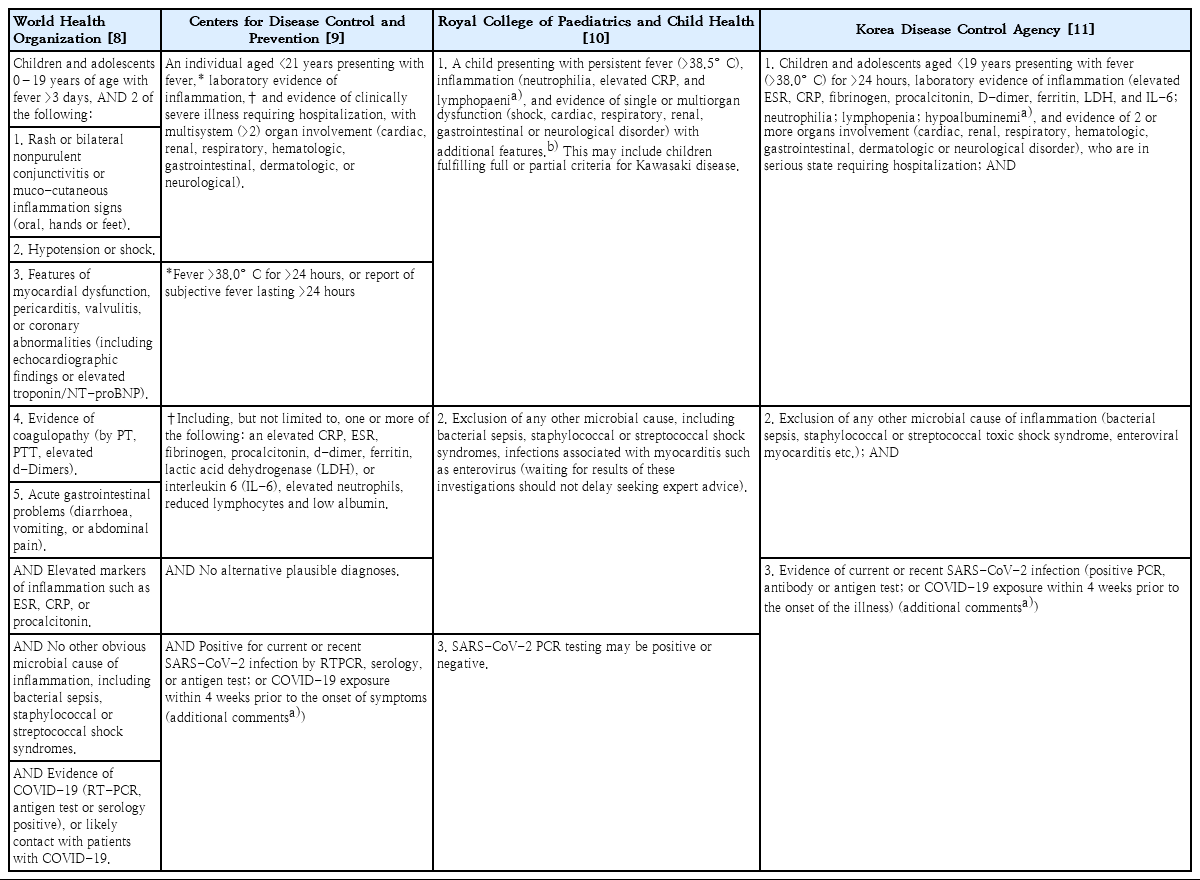
In May 2020, the World Health Organization, Centers for Disease Control and Prevention of the US, and Royal College of Paediatrics and Child Health (RCPCH) of the United Kingdom (UK) simultaneously published case definitions for MIS-C or PIMS-TS [8-10]. In Korea, the case definition was published in June 2020 by the Korea Disease Control and Prevention Agency (KDCA) [11]. Each case definition differs somewhat from the others, but doctors may diagnose patients with MIS-C without much difficulty using any one of them. However, the case definition by RCPCH seems less strict in requiring evidence of multiorgan involvement and SARS-CoV-2 infection (Table 1).
The principal symptoms and signs of MIS-C are similar to those of KD, which suggests a shared pathogenesis. Therefore, clinicians should suspect MIS-C, if patients present with fever, KD-like features (skin rash, conjunctivitis, oral mucosa changes, hand or foot edema), and/or gastrointestinal symptoms (abdominal pain, vomiting, diarrhea), and test positive for SARS-CoV-2 (by PCR or serology test) or have a history of recent contact with COVID-19 patients.
Epidemiology of MIS-C
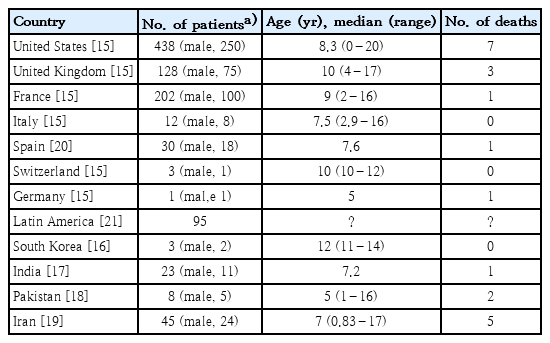
Hundreds of cases of children and adolescents with MIS-C have been reported first in Europe and the US since April 2020 during the COVID-19 pandemic [4-7]. They had evidence of SARS-CoV-2 infection (positive RT-PCR or serology test) or a history of recent contact with COVID-19 patients. Most reported patients with MIS-C were children and adolescents, whereas 27 adult patients with clinical features of multisystem inflammatory syndrome were reported in the US and UK, referred to as multisystem inflammatory syndrome in adults [12]. Most children affected by MIS-C were previously healthy without prior morbidities. MIS-C showed clinical features similar to KD, but their age, sex, and racial distributions differed somewhat from those of KD patients. KD usually affects infants and young children less than 5 years of age (approximately 80% are less than 5 years and about half are less than 2 years), and KD affects about 1.5 times more boys than girls [13,14]. In contrast, MIS-C equally affected children and adolescents (median age, 8.6 years; interquartile range, 7–10 years; range, 3 months–20 years) [15] and affected slightly more boys than girls [4-7,15]. The incidence of KD is markedly high in East Asian countries, including Japan, Korea, China, and Taiwan, but low in Europe and the US [13,14]. In contrast, most patients with MIS-C have been reported in Europe and the US [4-7,15], while some MIS-C cases have been reported in Korea, India, Pakistan, and Iran among Asian countries (Table 2) [15-21]. It is not yet known why the incidence of MIC-S is low in Asian countries. This may be due to the role of racial factors in the development of MIS-C, changes in the pathogenicity of SARS-CoV-2, or differences in population sizes affected by SARS-CoV-2 infection in Asia, Europe, and the US.
MIS-C usually develops 2–4 weeks after SARS-CoV-2 infection [4-7]. However, a few MIS-C cases were identified to develop later than 4 weeks after SARS-CoV-2 infection in epidemiological surveys. In addition, many MIS-C cases had negative PCR and positive antibody test results for SARS-CoV-2, implying that they would be in the early convalescent stage of SARS-CoV-2 infection [4-7,12]. In Korea, 2 of 3 reported cases of MIS-C seemed to develop later than 4 weeks after the diagnosis of or exposure to COVID-19, and one had negative PCR and positive antibody test results for SARS-CoV-2 [16].
Pathogenesis of MIS-C
The causal relationship between COVID-19 and MIS-C seemed uncertain despite many MIS-C cases developing during the COVID-19 pandemic. Because COVID-19 has been prevalent in Europe and the US, hyperinflammatory response syndromes may have developed concurrently as a complication of SARS-CoV-2 infection. In addition, MIS-C may be a group of heterogeneous diseases, including true cytokine storm syndrome (CSS) developing after SARS-CoV-2 infection, COVID-19 with severe inflammatory responses, and KD occurring concurrently with SARS-CoV-2 infection [7]. In contrast, SARS-CoV-2 infection might be a prerequisite for the development of MIS-C.
Patients diagnosed with MIS-C showed features of markedly increased inflammation with or without shock and cardiac dysfunction, which required differential diagnosis from toxic shock syndrome, KD, KD shock syndrome, or macrophage activation syndrome [4-7]. Therefore, MIS-C could be included within the spectrum of the systemic inflammatory response syndrome or CSS (or cytokine release syndrome) like KD, which might explain why it seemed similar to KD. Most MIS-C patients show clinical manifestations and laboratory findings similar to KD, so the pathogenesis of MIS-C and KD might be the same [22,23]. The pathogenesis of KD is explained by the theory of abnormal or dysregulated immune reactions. It has been postulated that one of pathogens (virus, bacteria, or fungus), toxins, or environmental agents may trigger abnormal or dysregulated immune reactions in genetically susceptible children, leading to the release of large amounts of inflammatory cytokines and cause KD manifesting as systemic vasculitis [13]. Therefore, KD can be included within the spectrum of CSS such as MIS-C. However, some clinicians consider MIS-C and KD different disease entities because their age and regional or racial distributions differ and MIS-C usually shows more severe clinical features than KD [5,24]. Fig. 1 shows the relationship between genetic susceptibility, triggering factors, and hyperinflammatory responses in the development of MIS-C.
1. Genetic susceptibility
The morbidity and mortality rates of COVID-19 are generally linked to patients’ old age and comorbidities [25,26]. However, a small proportion (about 5%) of COVID-19 patients developed severe lung injury or multiple organ dysfunction regardless of age [26,27], suggesting the role of genetic susceptibility in the pathogenesis of COVID-19 with severe inflammatory responses or MIS-C. The incidence of KD is much higher in East Asian countries (Japan, Korea, China, and Taiwan) than in Europe, the US, and other Western countries, which suggests that genetic susceptibility may play an important role in its pathogenesis [14]. In contrast, most MIS-C cases were reported in Europe and the US, and MIS-C affected more Black and Hispanic people than White people, suggesting that different genetic susceptibilities might play an important role in the pathogenesis of MIS-C [4-7].
2. Triggering factors
Pediatric immune-mediated diseases are generally triggered by infectious agents, drugs, environmental agents, or trauma [28,29]. Clinicians suspect MIS-C as a complication of SARS-CoV-2 infection because MIS-C cases were reported in a cluster during the COVID-19 pandemic but rarely reported before it [5,24]. In addition, many consider SARS-CoV-2 infection the infectious triggering factor inducing hyperinflammatory responses in MIS-C [4-7]. The causal relationship between SARS-CoV-2 infection and MIS-C remains unclear, but it may become evident by comparison of the change in incidence of MIS-C versus COVID-19. If the incidence of MIS-C is proportional to that of COVID-19, we could say that the 2 diseases are related to each other. In contrast, if the incidence of MIS-C is not proportional to that of COVID-19, we might say that the 2 diseases are not related.
3. Hyperinflammatory response
Many experts believe that CSS plays a key role in the development of MIS-C [24,27]. The manifestations of CSS were already observed in some adult patients with COVID-19 [30,31]. They were also observed in some patients with SARS in 2002, the Middle East respiratory syndrome in 2012, and the new influenza disease in 2009 [31-33]. CSS is considered a heterogeneous disease group that develops various symptoms and signs of varied severities according to triggering factors and host vulnerability [28-30].
Another mechanism may play a role in the development of MIS-C, that is, the postinfectious or delayed parainfectious mechanism [22,27]. Supporting evidence is provided below. MIS-C generally develops 2–4 weeks after SARS-CoV-2 infection instead of immediately after SARS-CoV-2 infection [4,24]. Many MIS-C patients had negative PCR and positive antibody test results for SARS-CoV-2, implying that they would be in the early convalescent stage of SARS-CoV-2 infection [4-7,12]. Most MIS-C patients had marked gastrointestinal symptoms (abdominal pain, vomiting, diarrhea) rather than respiratory symptoms, suggesting that SARS-CoV-2 might infect enterocytes secondarily and replicate in the gastrointestinal tract [34]. All of the above factors suggest the possibility of the role of autoantibody or immune complex in the pathogenesis of MIS-C, such as reactive arthritis, post-streptococcal glomerulonephritis, and rheumatic fever, which develop after viral or bacterial infections. Other possible mechanisms include bystander activation of nonspecific T cells, the production of auto-reactive immune cells by molecular mimicry, and antibody-dependent enhancement of immune reactions [33,34].
Clinical features of MIS-C
1. Symptoms and signs
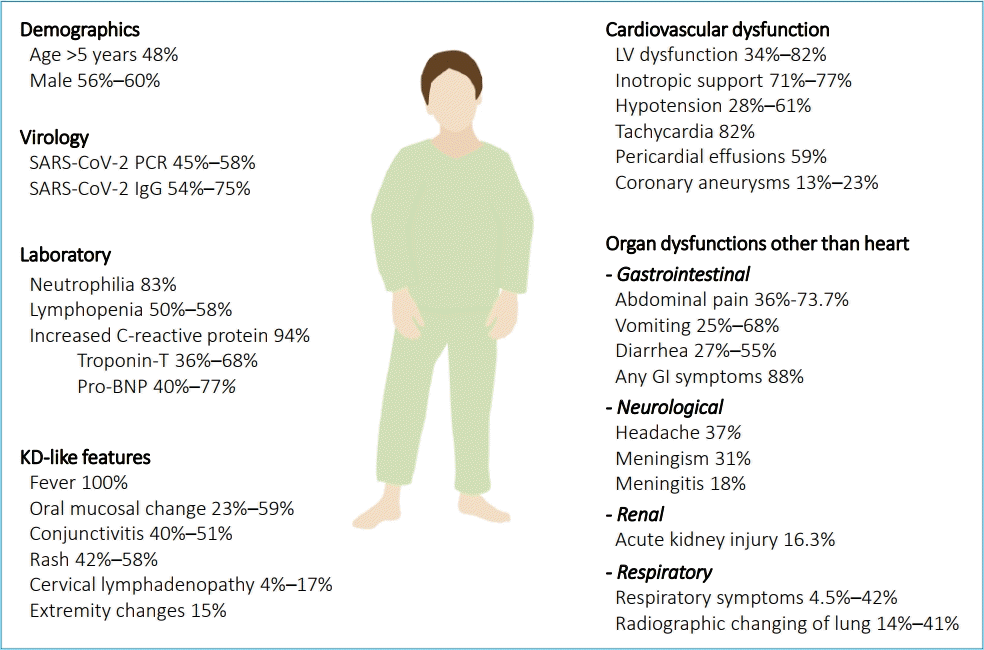
Most MIS-C patients present with KD-like symptoms and gastrointestinal symptoms in addition to fever with or without shock or cardiac dysfunction. Gastrointestinal symptoms mimicking viral gastroenteritis or mesenteric lymphadenitis were seen in up to 70% of patients (abdominal pain in 36%, vomiting in 25%, diarrhea in 27%) [5,35]. KD-like features were seen in many patients (skin rash in 42%–58%, oral mucosal changes [red fissured lips, strawberry tongue] in 23%–59%, conjunctival injection in 40%–51%, edema in hands and feet in 15%, and cervical lymphadenitis in 4%–17%). Cardiovascular abnormalities (myocardial dysfunction, valvular regurgitation, coronary ectasia or aneurysm, pericarditis, shock) were reported in 34%–82% of patients. Hypotension was reported in 28%–61% of patients. Respiratory symptoms (cough, sputum, tachypnea) were reported in a relatively small proportion of patients [15,36]. Therefore, the prominent difference in symptoms between MIS-C and COVID-19 was that skin rash and gastrointestinal symptoms were more common in MIS-C, whereas respiratory symptoms were more common in COVID-19 [37]. Similarities between MIS-C and KD were the presence of a high fever and the high prevalence of oral mucositis, conjunctivitis, and skin rash. However, gastrointestinal symptoms, cardiac dysfunction, and need for inotropic support were considerably more prevalent in MIS-C than in KD. In addition, a larger proportion of MIS-C patients developed shock than KD patients (28% vs. <3%) (Fig. 2) [38].
2. Results of SARS-CoV-2 tests
The positive rates of SARS-CoV-2 tests (RT-PCR, antibody, or antigen test) varied according to various reports and studies from Europe and the US. Approximately 45%–58% of reported patients with MIS-C had positive PCR test results for SARS-CoV-2, 54%–75% had positive antibody test results for SARS-CoV-2, and 7%–33% had positive test results for both [20,35,39,40]. A prior history of COVID-19 exposure was confirmed in 38%–52% of MIS-C patients [20,40]. Many children and adolescents seemed to develop MIS-C without a prior history of COVID-19 exposure, suggesting that asymptomatic spread of SARS-CoV-2 infection could have led to the development of MIS-C in many cases.
3. Laboratory findings
Inflammatory markers were elevated in all MIS-C patients. An increased CRP level or ESR was observed in all patients. The procalcitonin level was also increased in most patients, being much higher in MIS-C patients than in COVID-19 patients. Neutrophilia and lymphopenia were observed in most patients, and other hematologic abnormalities (anemia and thrombocytopenia) were observed in some patients. Increased ferritin levels (>500 ng/mL) were reported in more than half of patients, and increased D-dimer and fibrinogen levels have been reported in some patients [35-37]. Increased IL-6, IL-8, and tumor necrosis factor levels were reported in most patients, while IL-1 levels were reportedly normal. Increased cardiac marker levels were reported in a considerable number of patients: median B-type natriuretic peptide (BNP), 388 pg/mL (interquartile range [IQR], 75–1,086 pg/mL), median N-terminal pro-B type natriuretic peptide, 4328 pg/mL (IQR, 2,117–13,370 pg/mL), and median troponin T level, 0.08 ng/mL (IQR, 0.02–0.17 ng/mL) (Fig. 2) [4,20,37,39,41-43]. The elevated troponin level was an independent prognostic factor of a poor outcome [3].
On echocardiography, decreased left ventricular ejection fraction below 55% was reported in 32% of patients, of whom 11% had decreased ejection fraction below 30%. The evidence of myocarditis was present in 23% of patients. Among patients presenting with KD-like symptoms, 23.4% had coronary arterial dilatation or aneurysm [35-37]. On chest radiography or chest computed tomography, 13%–41% of patients showed pulmonary lesions, including opacities and infiltrates (Fig. 2) [35-37].
Treatments for MIS-C
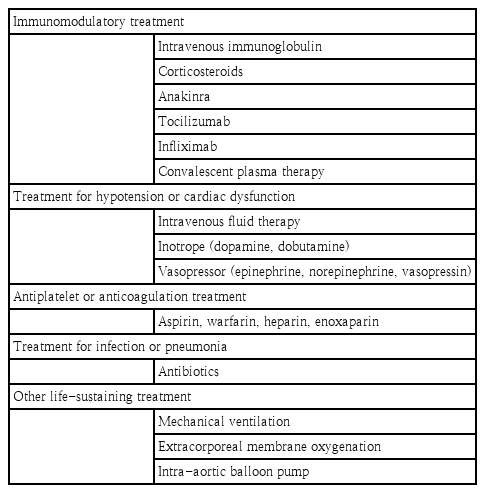
There are currently no widely accepted guidelines for the treatment of MIS-C, but treatment strategies generally follow the standard therapy of KD, including intravenous immunoglobulin (IVIG) and corticosteroids [35-37]. This is because overlapping characteristics between the 2 illnesses suggested they might share a similar pathophysiology and respond to similar treatments. High-dose IVIG (1–2 g/kg) and corticosteroids (low to medium dose) are generally recommended for the treatment of MIS-C. High-dose corticosteroids (methylprednisolone pulse therapy) may be considered for treating refractory patients or patients with life-threatening complications such as shock [44,45]. Some patients required a second dose of IVIG due to persistent fever [42,46].
Some refractory patients received monoclonal antibodies to the IL-6 receptor (tocilizumab), IL-1 receptor antagonist (anakinra), monoclonal antibodies to tumor necrosis factor (infliximab), or convalescent plasma therapy. One of 3 MIS-C patients in Korea had a persistent fever after the second dose of IVIG and intravenous corticosteroid treatment, and eventually received anakinra with resolution of fever [16]. Anakinra has a short half-life and quick onset of action, allowing rapid discontinuation of therapy in cases of adverse reactions [47]. Anakinra (>4 mg/kg/day IV or subcutaneous injection) is recommended for patients whose MIS-C is refractory to IVIG and/or corticosteroid treatment. The liver function of children who have received anakinra should be monitored. Tocilizumab (<30 kg: 12 mg/kg IV; ≥30 kg: 8 mg/kg IV; max.: 800 mg) may effectively reduce mortality and the need for intensive care unit admission in patients with severe COVID-19 pneumonia and signs of hyperinflammation. However, patients treated with tocilizumab may be at higher risk of developing bacterial or fungal infections [45]. There is no consensus on which of these agents is optimal, and drug choice may depend on the clinician’s preference, cytokine test results, and drug availability.
Aspirin (for anti-inflammation or prophylaxis of thrombosis) and/or anticoagulants are commonly administered to children with MIS-C [48]. Low-dose aspirin (3–5 mg/kg per oral medication once daily) is recommended in MIS-C patients with KD-like features, coronary aneurysm, or thrombocytosis. Anticoagulation therapy with heparin, enoxaparin, or warfarin is recommended for MIS-C patients with a giant coronary aneurysm (diameter >8 mm) or a coronary artery z score >10. MIS-C Patients with documented thrombosis or ejection fraction <35% need therapeutic doses of enoxaparin for at least 2 weeks after hospital discharge [45].
Hypotension in children with MISC is often resistant to intravenous fluid therapy, so inotropes and/or vasopressors should be used appropriately in such cases. Epinephrine is recommended as the first choice in children, and norepinephrine should be used if shock persists. The use of dobutamine or dopamine is recommended in patients with myocardial dysfunction because of its inotropic effect [49-51]. Other life-sustaining treatments, such as mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and a intra-aortic balloon pump, have been used in some patients admitted to the intensive care unit [35,39]. The empirical use of broad-spectrum antibiotics for possible pneumonia or sepsis has been reported in many studies [35-37,39]. Because of clinical and biochemical similarities of MIS-C with KD, the principle of therapy for KD was adapted to MIS-C and associated with rapid clinical improvement and reduced inflammatory marker levels in most patients. Table 3 summarizes the currently recommended treatments for MIS-C.
Outcomes of MIS-C
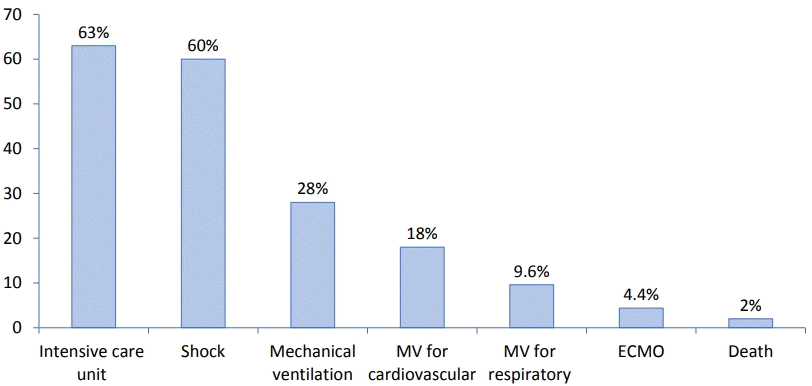
According to a systematic review, the duration of hospitalization was 4–13 days (median, 7 days), and intensive care was required in 68% of patients [35]. Inotropic support was required in 40%, mechanical ventilation was required in 15%, and ECMO was required in 2.7% [35]. The fatality rate was reportedly 1.7% in the US and 1.4% in Europe [35]. Among the studies that reported outcomes at discharge [20,39] or during follow-up [41,42], almost all patients with cardiac involvement experienced nearly full recovery of left ventricular function and normalization of cardiac inflammatory markers except for mild cardiac dysfunction observed in 9 patients at discharge in one study [43]. However, the long-term prognosis of MIS-C remains unknown. For example, long-term follow-up is necessary for coronary arterial aneurysm if present. In addition, follow-up studies and long-term cardiac surveillance are needed to monitor cardiac function and coronary arterial abnormalities. Fig. 3 summarizes the overall clinical outcomes of MIS-C.
Conclusion
Hundreds of cases of children and adolescents with MIS-C have been diagnosed during the COVID-19 pandemic. These patients showed clinical features similar to KD or CSS, so MIS-C may be within the spectrum of CSS. Clinicians should suspect MIS-C if patients present with fever, KD-like features (skin rash, conjunctivitis, oral mucosa changes, hand or foot edema), and/or gastrointestinal symptoms (abdominal pain, vomiting, diarrhea) and demonstrate evidence of SARS-CoV-2 infection. Despite accompanying shock or cardiac dysfunction, most MIS-C patients responded well to treatments and recovered without sequelae if adequate treatments were given. The fatality rate was approximately 1.5%–2%. As the COVID-19 pandemic might persist for a long time, we will likely see more MIS-C patients. In Korea, the KDCA constructed a surveillance system for MIS-C through a consultative process with academic societies and clinicians, and we need to be continuously vigilant about ensuring the early diagnosis and treatment of patients with MIS-C.
Notes
No potential conflict of interest relevant to this article was reported.