Eosinophils and childhood asthma
Article information
Abstract
Eosinophils are a type of granulocyte with eosinophilic granules in the cytoplasm that play an important role in allergic and parasitic diseases. Eosinophils are important in the pathogenesis of asthma, and many studies have examined the relationship between them. In allergic eosinophilic asthma, eosinophils act not only as important effector cells but also as antigen-presenting cells in allergic inflammatory reactions. In nonallergic eosinophilic asthma, type 2 innate lymphoid cells in the airways play an important role in eosinophil activation. Direct methods, including bronchial biopsy, bronchoalveolar lavage, and the induced sputum test, are used to evaluate eosinophilic inflammatory reactions in patients with asthma, however, because of difficulty with their implementation, they are sometimes replaced by measurements of blood eosinophils, fraction of exhaled nitric oxide, and serum periostin level. However, these tests are less accurate than direct methods. For the treatment of patients with severe eosinophilic asthma, anti-interleukin-5 preparations such as mepolizumab, reslizumab, and benralizumab have recently been introduced and broadened the scope of asthma treatment. Although eosinophils are already known to play an important role in asthma, we expect that further studies will reveal more details of their action.
Key message
∙ In allergic eosinophilic asthma, eosinophils act as important effector cells and antigen-presenting cells, while in nonallergic eosinophilic asthma, type 2 innate lymphoid cells play an important role in eosinophil activation.
∙ Sputum eosinophil counts can be helpful for evaluating allergic airway inflammation in asthma.
∙ Anti-interleukin-5 has broadened the scope of asthma treatment.
Introduction
Asthma is a chronic inflammatory disease of the airways associated with reversible airway obstruction, bronchial hypersensitivity, and varying degrees of symptoms [1]. About 300 million people worldwide have asthma, with an estimated 2 million in Korea [2,3]. The different asthma subtypes result from the complex interaction among several genetic and environmental factors and present as multiple phenotypes [4]. In the pathogenesis of asthma, T cells, especially T helper 2 (Th2) cells, play an important role in the initiation and maintenance of inflammation. Recent studies suggest that there are at least 2 endotypes of asthma, T2 “high” and T2 “low,” according to the extent of Th2-mediated inflammation [5,6]. The exact role of eosinophils in asthma remains controversial [7-10]. Eosinophilia does not increase in all cases of asthma; it is more common in allergic asthma but may also increase in nonallergic asthma [11]. The recent use of eosinophil-related anti-interleukin (IL) 5 agents in clinical practice may require further understanding of the role of eosinophils in asthma and help elucidate the role of eosinophils. This review discusses the relationship between asthma and eosinophils.
Eosinophils
Eosinophils, a type of granulocyte with eosinophilic granules in the cytoplasm, account for 1%–3% of white blood cells in the blood of healthy people. They are slightly larger than red blood cells [12,13]. The level of eosinophils in the blood is highest in the morning and lowest in the afternoon. The nucleus is composed of 2 connected lobes, and the cytoplasm is filled with granules that stain red on hematoxylin and eosin staining. Eosinophils are produced in the bone marrow and circulate in the blood for approximately 18 hours, then migrate to the tissues and are present primarily in the tissues (>95%). Tissue eosinophils are generally distributed in the gastrointestinal mucosa, particularly the small intestine, and some are found in the spleen and lymph nodes. Eosinophils are believed to survive for more than 8–12 days in the tissues, but data are limited. Eosinophils generally do not recirculate and are discharged or dissolved in the intestinal lumen [13]. Their granules can cause tissue damage or dysfunction. In addition, eosinophils secrete inflammatory cytokines, such as IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, IL-16, IL-18, and transforming growth factor α/β (TGF-α/β); chemokines such as RANTES (regulated on activation, normal T cell expressed and secreted) and eotaxin 1; and lipid mediators such as platelet-activating factors and leukotriene C4 [14]. Eosinophils are involved in the pathophysiology of various diseases, including parasitic and allergic diseases [15]. Eosinophils move from the blood to inflammatory sites, perform multiple functions by directly controlling cytokines and inflammatory responses, and act as antigen-presenting cells in inflammatory responses.
Eosinophilic and noneosinophilic asthma
Eosinophilic asthma (EA) features a significant number of eosinophils in the airway and/or blood, whereas non-EA (NEA) features a small number of eosinophils [16]. EA is diagnosed on the induced sputum test when eosinophils comprise more than 2%–3% of the total cells in a sample [17]. Inhaled corticosteroids are preferred to other drugs for treatment of EA [18,19]. In severe cases of EA that do not respond well to high-dose inhaled or systemic steroids, eosinophil-related biologics may be considered. EA can be divided into allergic and nonallergic EA. Allergic EA, also known as general EA, is associated with eosinophilia, part of the Th2 allergic inflammatory response, while nonallergic EA is caused by a mechanism other than allergic inflammation. The mechanisms are discussed in the next section, “pathogenesis of asthma and eosinophils.”
In recent studies, asthma phenotypes were classified into several clusters rather than EA versus NEA. In the cluster analysis of the Severe Asthma Research Program cohort, the children were divided into 4 childhood asthma clusters (late-onset symp tomatic asthma, early-onset atopic asthma with normal lung function, early-onset atopic asthma with mild airflow limitation, and early-onset atopic asthma with advanced airflow limitation) according to asthma duration, baseline lung function, and number of controller drugs required. There were various levels of blood eosinophils in all 4 clusters but no significant intergroup differences [20]. In the cluster study of the Unbiased Biomarkers for the Prediction of Respiratory Disease Outcome Consortium cohort, asthma patients were divided into 4 clusters, 3 of which were associated with eosinophilia [21].
Pathogenesis of asthma and eosinophils
In allergic EA, eosinophils are important effector cells of allergic inflammatory reactions. Their activation results in the extracellular release of granular proteins such as eosinophil cationic protein (ECP), eosinophil-derived neurotoxin, major basic protein (MBP), and eosinophil peroxidase (EPO) [22]. Among the various mediators secreted by eosinophils, these granules play the most powerful and important role in the pathophysiology of asthma [23]. Eosinophils also release a number of cytokine, chemokine, and lipid mediators. In allergic inflammatory reactions, eosinophils have been considered terminal effector cells, but recent studies have shown that they are also involved in the early stages of the development of allergic diseases [24]. Eosinophils can act as antigen-presenting cells, inducing T-cell activation and cytokine production in early disease stages [25,26].
In nonallergic EA, type 2 innate lymphoid cells in the airways are thought to play an important role and be activated by IL-25, IL-33, and prostaglandin D2 [27]. Type 2 innate lymphoid cells are a local source of IL-5 and IL-13, which are important for eosinophil activation and are associated with eosinophilia in the airways and the occurrence of severe asthma [28].
In connection with airway remodeling in asthma, activated eosinophils secrete several profibrogenic molecules. ECP increases proteoglycan production in fibroblast cell cultures [29], increases TGF-β production in fibroblasts [30], and enhances collagen gel contraction [31]. Eosinophils also produce TGF-β, a powerful profibrogenic cytokine, on their own. EPO and MBP induce airway epithelial cells to produce TGFs, matrix metalloproteinases, and platelet-derived growth factors [32]. In one study, a decrease in eosinophils with anti-IL-5 administration decreased subepithelial fibrosis in the airways [33].
Laboratory tests associated with eosinophils (eosinophil biomarkers) in asthma
Methods for directly evaluating eosinophilic inflammation of the airways include bronchial biopsy, bronchoalveolar lavage, and the induced sputum test. Bronchial biopsy and bronchoalveolar lavage have been considered the gold standards for evaluating eosinophilic inflammation of the airways [34]. However, because these tests are very invasive, the induced sputum test is the most widely used [35].
Sputum eosinophil counts decrease with corticosteroid treatment and are increased by allergen challenge [36]. Monitoring sputum eosinophil counts showed reduced exacerbation numbers and severities in adult patients [37]. However, data are lacking for the same in children. Fleming et al. [38] reported that monitoring induced sputum in children did not significantly reduce overall exacerbations or improve asthma control. In this study, follow-up every 3 months revealed that exacerbations were reduced in the short term, suggesting that more frequent measurements would be needed to confirm a clinically useful effect.
There are some limitations to the induced sputum test. It is almost impossible to collect sputum from children under the age of 5 years, and the hypertonic saline used to induce sputum is likely to cause asthma attacks in asthmatic patients. In addition, the results of the sputum test are not immediately available after the samples have been obtained because skilled inspectors are required to examine the samples, which takes time. Accordingly, measurements of blood eosinophils, the fraction of exhaled nitric oxide (FeNO), and serum periostin level determination may be performed in the clinic instead of the induced sputum test to evaluate eosinophilic inflammation in the airways [39].
Blood eosinophil count can be easily performed at a lower cost than the induced sputum test. Despite a correlation between blood and sputum eosinophils, use of the former rather than latter has been controversial due to high false negative and positive rates [40]. Blood eosinophils are useful for screening patients with asthma who will respond well to biological agents such as anti-IL-5 [39].
FeNO is a gaseous molecule synthesized by nitric oxide synthase that is expressed in the airway epithelium and increases in the inflammatory state. Measuring FeNO is a noninvasive method for evaluating airway inflammation in asthma. The test is relatively simple and its results are immediately available [41]. Many studies have shown a positive relationship between FeNO and sputum eosinophil count [42,43], and FeNO has been proposed as a test for monitoring asthma [44].
Serum periostin is a recently introduced test that is used to indirectly evaluate eosinophilic airway inflammation in patients with asthma. In several reports, serum periostin levels were significantly correlated with sputum eosinophil counts [45,46]. Woodruff et al. [47] reported that the asthma group showed higher periostin gene expression in the bronchoalveolar lavage fluid and higher concentrations of IL-4, IL-5, and IL-13 than the control group as well as increased eosinophil concentrations in the bronchoalveolar lavage fluid. Wagener et al. [48] reported that serum periostin had lower accuracy than blood eosinophils and FeNO for evaluating eosinophilic inflammation in asthmatic patients.
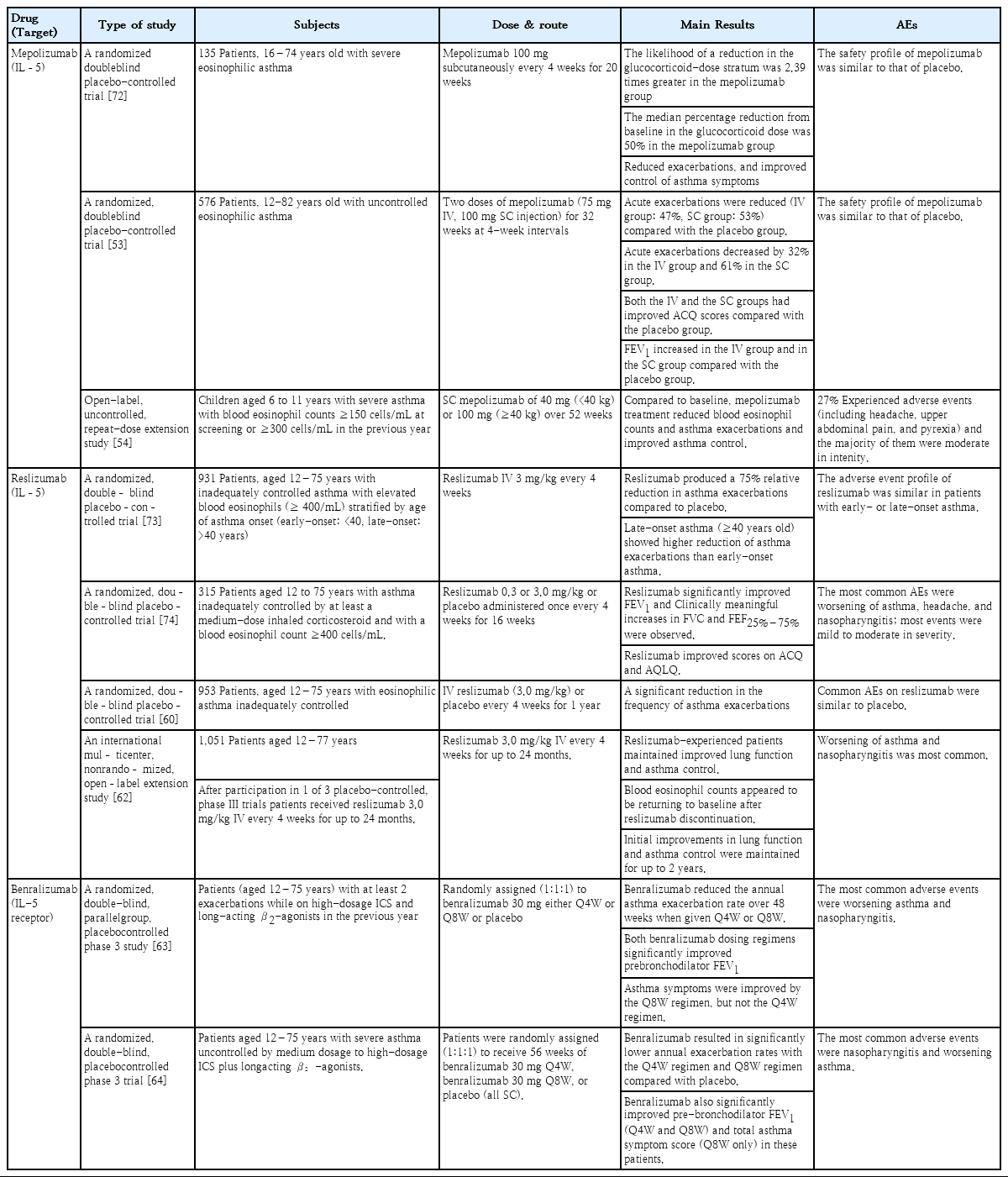
Clinical studies on eosinophils and childhood asthma other than antieosinophil drugs summarized in Table 1.
Antieosinophil (anti-IL-5) drugs and asthma
IL-5 is a cytokine secreted by lymphocytes, mast cells, and eosinophils that is highly correlated with eosinophil differentiation, proliferation, and activation [49]. Thus, it is an important target in the consideration of biological treatment in EA patients. Anti-IL-5 therapy was introduced in the late 1990s based on animal studies that showed that airway hyperresponsiveness was suppressed and eosinophil counts in bronchoalveolar lavage fluid were reduced by anti-IL-5 therapy [50]. However, these findings did not demonstrate clinical success since pulmonary function improvement was the target. Anti-IL-5 therapy has attracted attention again from recent positive reports of its effectiveness with strengthened screening of target patients, and it is listed in asthma guidelines as a treatment for severe EA. Currently available anti-IL-5 agents include mepolizumab, reslizumab, and benralizumab (Table 2).
Mepolizumab
Mepolizumab (Nucala; GlaxoSmithKline, London, UK) is a humanized monoclonal antibody (IgG1) specific for IL-5 that has been approved by the U.S. Food and Drug Administration (FDA) for further treatment of patients aged 12 years or older with severe EA. Mepolizumab selectively inhibits eosinophilic inflammation and reduces the number of eosinophils in the sputum and blood [51]. In a randomized double-blind placebocontrolled study of 61 patients, mepolizumab reduced the frequency of exacerbations in patients with severe EA with repeated exacerbations despite the use of high-dose steroids (P=0.02) and reduced eosinophil counts in the peripheral blood and sputum (P<0.001) compared with the placebo group [52]. However, mepolizumab did not improve forced expiratory volume in 1 second (FEV1) or airway hyperresponsiveness. In a small study, mepolizumab treatment reduced the use of systemic steroids in patients who had severe EA with uncontrolled symptoms despite the use of systemic steroids and high-dose inhaled steroids by 47.7% versus placebo (P=0.04) [51]. In the Steroid Reduction with Mepolizumab Study, mepolizumab reduced the steroid dose by 50% in asthma patients with increased peripheral blood eosinophils and were receiving systemic steroids. In addition, the rate of acute exacerbation was 32% lower (P=0.04) and the Asthma Control Questionnaire (ACQ) score was also lower than that in the placebo group despite a significant reduction in steroid dose (P=0.04). However, 3 months after treatment with mepolizumab was stopped, the eosinophilia in the blood and sputum returned to its original state. In the Mepolizumab as Adjunctive Therapy in Patients with Severe Asthma study, which included children aged 12 years or older in addition to adults [53], 2 doses of mepolizumab (75 mg intravenous, 100 mg subcutaneous injection) were administered for 32 weeks at 4-week intervals to EA patients who had uncontrolled and repeated acute exacerbations despite the use of high-dose inhaled steroids. Acute exacerbations were reduced by 47% in the intravenous group and by 53% in the subcutaneous group compared with the placebo group (P<0.001). Acute exacerbations requiring emergency visits or hospitalization decreased by 32% (P=0.03) in the intravenous group and by 61% (P=0.02) in the subcutaneous group. The intravenous and subcutaneous groups had improved ACQ scores compared with the placebo group (P<0.001). The mean FEV1 increased by 100 mL (P=0.02) in the intravenous group and 98 mL (P=0.03) in the subcutaneous group versus the placebo group. In a study by Gupta et al. [54], children aged 6–11 years with severe asthma and blood eosinophil counts ≥150 cells/mL at screening or ≥300 cells/mL in the previous year were enrolled. They received subcutaneous mepolizumab (40 mg, <40 kg) or 100 mg (≥40 kg) over 52 weeks. Compared to baseline, mepolizumab treatment reduced blood eosinophil counts and asthma exacerbations and improved asthma control. Twenty-seven percent of patients experienced adverse events related to mepolizumab treatment, including headache, upper abdominal pain, and pyrexia, although the majority were moderate in intensity.
Reslizumab
Reslizumab (Cinqair; Teva Respiratory, Frazer, PA, USA) is an IgG4 humanized monoclonal antibody against IL-5 that has been approved by the FDA as a treatment for severe EA in patients 18 years of age or older. Reslizumab is generally recommended to be administered at a dose of 3 mg/kg intravenously every 4 weeks [55]. In a phase 3 study of reslizumab, improvements in lung function did not differ from the placebo group when the blood eosinophil count was less than 400 cells/mL [56]. Mukherjee et al. [57] found that fixed-dose mepolizumab did not significantly affect steroiddependent asthma, whereas weight-based reslizumab treatment had superior ability to reduce sputum eosinophils and improve asthma control compared with placebo. In a study by Castro et al. [58], serum and sputum eosinophils decreased and FEV1 improved in the group administered reslizumab 3 mg/kg for 15 weeks compared with the placebo group. There was no significant intergroup difference in ACQ score, but among patients with a nasal polyp, the reslizumab group had a higher ACQ score than the placebo group. Although large-scale studies on the effect of reslizumab on steroid dose reduction in asthma patients who have been using oral steroids long term are lacking, reslizumab effectively improved asthma control and lung function in prednisone-dependent patients [59]. Another study reported a significant decrease in the frequency of asthma exacerbations in patients receiving reslizumab versus the placebo group [60].
In one study that included children aged 12 years or older with elevated blood eosinophils (≥400/mL) and inadequately controlled asthma, reslizumab produced a 75% relative reduction in asthma exacerbations compared to placebo. However, late-onset asthma (≥40 years old) showed a higher reduction in asthma exacerbations than early-onset asthma (<40 years old) [61].
In a phase 3 trial of patients with moderate-to-severe EA (blood eosinophils ≥ 400/mL) who were aged 12 years or older, reslizumab 3 mg/kg was injected intravenously every 4 weeks for up to 24 months. Reslizumab showed favorable long-term safety, and the initial improvements in FEV1, forced vital capacity, and forced expiratory flow at 25%–75% of the pulmonary volume were maintained for up to 2 years [62].
Benralizumab
Benralizumab (Fasenra; AstraZeneca, Cambridge, UK), a humanized IgG1 monoclonal antibody that binds to the IL-5 receptor α subunit of eosinophils and basophils, has been approved by the FDA for further treatment in patients with severe EA aged 12 years or older. When the antibody is bound, it causes cellular apoptosis through cell-mediated cytotoxicity. The 3 main placebo-controlled studies of benralizumab in patients with severe uncontrolled EA are the SIROCCO, CALIMA, and ZONDA trials.
In the SIROCCO trial, 1,205 patients were administered benralizumab or placebo for 48 weeks. The study included children aged 12 years or older as well as adults. Asthma exacerbations were reduced and FEV1 was improved in the benralizumab-administered group versus the control group [63]. The CALIMA trial included 1,306 patients over 56 weeks. Similarly, asthma exacerbation was reduced and FEV1 was improved in the benralizumab-treated group [64]. In the ZONDA trial, 220 subjects were enrolled for 28 weeks. Oral corticosteroid needs were reduced by 75% in the benralizumab-treated group and by 25% in the placebo group (P<0.001). Asthma exacerbations were also significantly reduced in the benralizumab-treated group [65].
Conclusion
Eosinophils are important in the pathogenesis of asthma and useful for evaluating asthma. With the recent introduction of anti-IL-5 agents that suppress eosinophils, the scope of asthma treatment has been broadened, and these medications are helpful for patients with severe EA. Although the role of eosinophils in asthma has been investigated in many studies, further studies are needed to deepen our understanding of asthma.
Notes
No potential conflict of interest relevant to this article was reported.