Clinical implications of coronavirus disease 2019 in neonates
Article information
Abstract
Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019, a small number of coronavirus disease 2019 (COVID-19) cases in neonates have been reported worldwide. Neonates currently account for only a minor proportion of the pediatric population affected by COVID-19. Thus, data on the epidemiological and clinical features of COVID-19 in neonates are limited. Approximately 3% of neonates born to mothers with COVID-19 reportedly tested positive for SARS-CoV-2. Current limited data on neonates with COVID-19 suggest that neonatal COVID-19 shows a relatively benign course despite a high requirement for mechanical ventilation. However, neonates with pre-existing medical conditions and preterm infants appear to be at a higher risk of developing severe COVID-19. The greatest perinatal concern of the COVID-19 pandemic is the possibility of vertical transmission, especially transplacental transmission of SARS-CoV-2. Although direct evidence of the vertical transmission of SARS-CoV-2 is lacking, its possibility during late pregnancy cannot be ruled out. This review summarizes available case studies on COVID-19 in neonates and introduces what is currently known about neonatal COVID-19 with focus on its vertical transmission.
Key message
• Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was detected in approximately 3% of neonates of mothers with coronavirus disease 2019 (COVID-19).
• Neonatal COVID-19 is relatively benign with 16%–22.4% cases asymptomatic.
• Neonates with pre-existing medical conditions and preterm infants are at a higher risk of severe COVID-19.
• Requirement for neonatal mechanical ventilation is 20%–22.4% (vs. 4% in children)
• Low birth weight (13.9%) and premature birth (22.2%) affect neonates of mothers with COVID-19.
Introduction
In December 2019, a pneumonia caused by a novel coronavirus that was later named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, Hubei Province, China. The outbreak of SARS-CoV-2 infection has resulted in a pandemic that has rapidly spread worldwide and has become the most serious public health threat [1,2]. Globally, as of February 1, 2021, there were more than 102 million confirmed cases of coronavirus disease 2019 (COVID-19) that caused more than 2.2 million deaths according to the World Health Organization (WHO) [3]. Although numerous studies have reported on COVID-19 in adults, there is a relative paucity of data on pediatric COVID-19, particularly among neonates. To date, a small number of neonatal COVID-19 cases have been reported worldwide. Since the report of the first neonatal case on March 29, 2020, several neonatal cases of COVID-19 have been reported in Korea [4]. One of the most important concerns of neonatal COVID-19 is whether or how SARS-CoV-2 can be transmitted from the mother to the fetus. The clinical course of COVID-19 in neonates is also of great interest.
Here the author summarizes available case studies of COVID-19 in neonates and reviews what is currently known about neonatal COVID-19 with a focus on vertical transmission
Burden of COVID-19 in the pediatric population
In Korea, as of February 1, 2021, a total of 78,508 patients had confirmed COVID-19, of whom 8,237 (10.5%) were children aged ≤19 years and 3,004 (3.8%) were children aged ≤9 years. No deaths from COVID-19 in children have been reported in Korea [5].
In the United States (US) and globally, there are fewer cases of COVID-19 in the pediatric population than in the adult population. While children aged 0–17 years account for 22% of the US population, recent data showed that cases of COVID-19 in children accounted for 11.1% of all COVID-19 cases in the US (as of February 1, 2021). Furthermore, COVID-19 cases in children aged ≤4 years accounted for 1.9% of all confirmed cases of COVID-19 in the US Children with COVID-19 are less likely to develop severe illness than adults. Deaths among children due to COVID-19 accounted for less than 0.1% of total deaths in the US [6,7]. In a Chinese nationwide study, when 2,143 children with COVID-19 were classified as asymptomatic, mild, moderate, severe, and critical cases based on COVID-19 severity, infants aged <1 year accounted for the highest proportion (10.6%) of severe and critical cases but the lowest proportion (1.8%) of asymptomatic cases among all age groups [8]. Among children aged <18 years with COVID-19 in the US between February 12 and April 2, 2020, infants aged <1 year accounted for the highest proportion (62% and 5.2%, respectively) of hospitalizations and intensive care unit (ICU) admissions among children with COVID-19 [9]. Taken together, these results suggest that infants aged <1 year might be at an increased risk of severe illness from COVID-19 compared to other children. However, COVID-19 severity in the neonatal group was not independently investigated in the epidemiological data from the US and China.
Clinical features of COVID-19 in neonates
Neonates currently account for only a minor proportion of the pediatric population affected by COVID-19. Thus, data on the epidemiological and clinical features of COVID-19 in neonates are limited, suggesting that neonatal COVID-19 is relatively benign despite concerns about the possibility of vertical transmission [10,11].
Among 322 neonates born to mothers with COVID-19, 10 (3.1%) tested positive for SARS-CoV-2 but did not die [10]. In a literature review of 25 neonates with COVID-19 from December 2019 to April 27, 2020, cesarean section occurred in 16 (64%) with a male-to-female ratio of 2.8 [11]. The mean gestational age and birth weight of affected neonates were 37.4 weeks (range, 26.6–41.3 weeks) and 3,042 g (range, 960–4,440 g), respectively. The mean age at onset was 8.2 days (range, 1–25 days). Affected neonates manifested fever (28%), vomiting (16%), and cough or shortness of breath (12%) at onset, while only 16% of affected neonates were asymptomatic. Major complications were pneumonia (12%), respiratory distress (8%), and sepsis (4%). Deaths were not reported. Among all neonates with COVID-19, intensive care and mechanical ventilation were required for 32% and 20% of cases, respectively. The diagnosis of COVID-19 was made at a mean 3.1 days after admission (range, 1–15 days) mainly by reverse transcription polymerase chain reaction (RT-PCR) from nasopharyngeal swabs. RT-PCR results were negative within a mean 10.3 days after diagnosis (range, 6–17 days) [11].
Classification of COVID-19 in neonates
A detailed classification system is likely to be helpful for selecting appropriate diagnostic methods and interpreting results for SARS-CoV-2 infection in neonates. A classification system and case definition of SARS-CoV-2 infection in pregnant women, fetuses, and neonates were recently proposed by Shah et al. [12]. In this classification system, maternal-fetal-neonatal SARS-CoV-2 infection was classified as follows: (1) maternal infection during pregnancy; (2) congenital infection with intrauterine fetal death/stillbirth; (3) congenital infection in live born neonate; (4) neonatal infection acquired intrapartum; and (5) neonatal infection acquired postpartum. Five categories were also adopted according to the likelihood of infection: (1) confirmed, (2) probable, (3) possible, (4) unlikely, and (5) not infected. The case definition of the classification system considered results of maternal and neonatal testing with the neonate’s clinical status. This classification system can be revised based on additional clinical data and more reliable diagnostic tests.
Summary of case studies of neonatal COVID-19
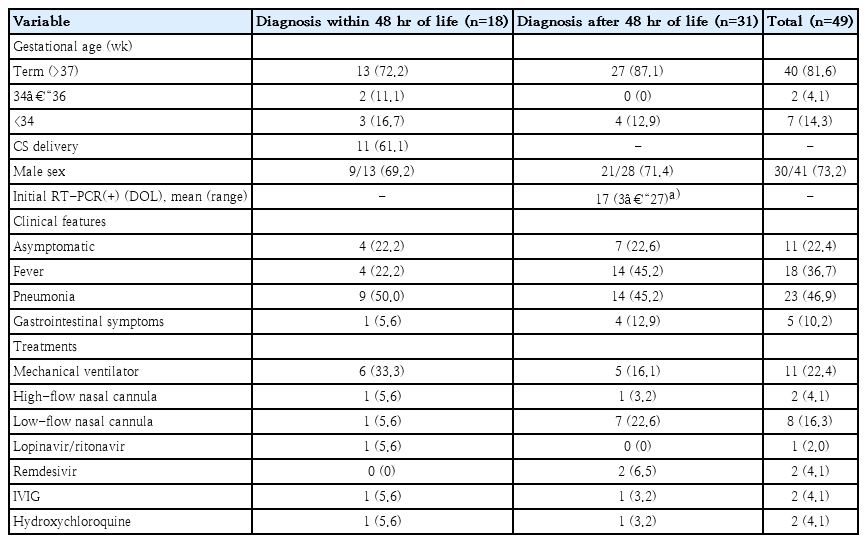
A comprehensive literature search and review for COVID-19 in neonates in articles published between December 2019 and September 2020 was conducted by single reviewer using PubMed (National Library of Medicine, Washington DC, USA). The terms used for the literature search were “COVID-19,” “SARS-CoV-2,” “neonates,” and “newborns.” Articles in English describing SARS-CoV-2–positive neonates at ≤28 days of life were included. Two extremely low gestational age neonates (ELGANs) diagnosed before 40 weeks’ postmenstrual age during routine intensive neonatal care were included, although they were diagnosed after 28 days of life. Articles were excluded if they did not address demographic features, clinical features, or outcomes of neonates with COVID-19. A total of 34 articles including 49 neonates with laboratory-confirmed COVID-19 were considered eligible for review. Twelve cases (the largest number of patients) were reported in the US and 9 cases were reported in China and Italy. Details of the neonates with COVID-19 are described in Tables 1 and 2 [4,13-44]. According to the definition by Shah et al. [12], the neonates with COVID-19 were divided into 2 groups. The first group consisted of 18 neonates diagnosed within 48 hours of life, suggesting the possibility of congenital or intrapartum infection (Table 1). The second group of 31 neonates was diagnosed after 48 hours of life; such infections were presumed to be acquired postpartum (Table 2).

Clinical details of 18 neonatal cases of COVID-19 diagnosed within 48 hours of life from 16 case studies
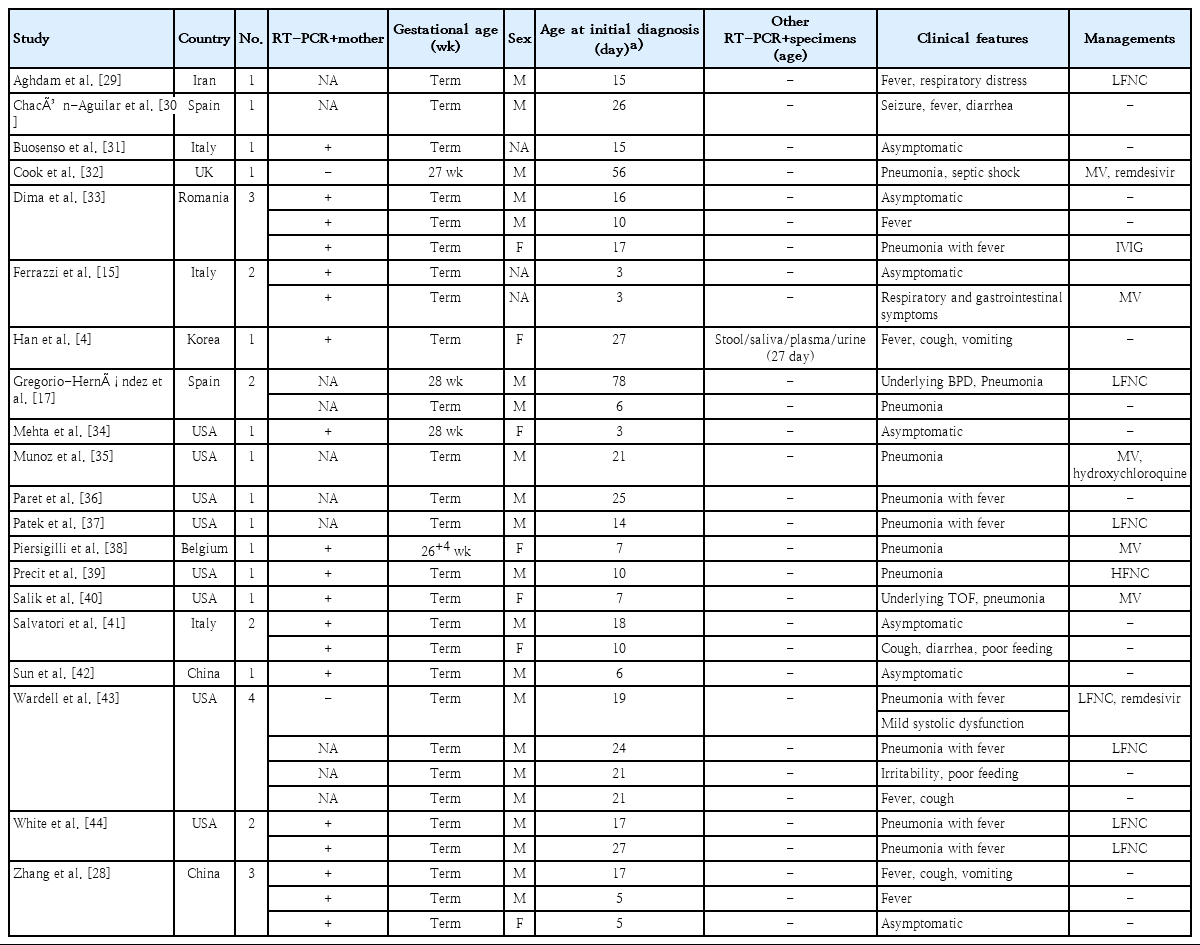
Clinical details of 31 neonatal cases of COVID-19 diagnosed after 48 hours of life from 20 case studies
The clinical features and management of neonates included in the present study are summarized in Table 3. A total of 49 neonates with COVID-19 were included. Of these, 40 (81.6%) were term (≥37 weeks). The youngest gestational age was 26 weeks and 4 days. The mode of delivery was available for neonates diagnosed within 48 hours of life. Cesarean section (61.1%) was more frequent than vaginal delivery. Neonatal SARS-CoV-2 infection was more common in male infants (73.2%). Among neonates diagnosed after 48 hours of life (except for the 2 ELGANs), the mean age at diagnosis was 17 days (range, 3–27 days). All neonates survived without any sequelae. Of all neonates with COVID-19, 22.4% were asymptomatic. In a multinational multicenter cohort study performed in Europe, approximately 16% of children with COVID-19 were asymptomatic [45]. Thus, the proportion of asymptomatic neonates (22.4%) in the present review was slightly higher than that reported in the literature. Neonates with COVID-19 manifested fever (36.7%), pneumonia (46.9%), and gastrointestinal symptoms (10.2%) including vomiting, diarrhea, and feeding intolerance. Of note, neonates with pre-existing medical conditions and preterm infants appeared to be at a higher risk of severe illness from COVID-19. Eleven neonates (22.4%) required mechanical ventilation. Other specific treatments used included administration of lopinavir/ritonavir (n=1), remdesivir (n=2), immunoglobulin (n=2), and hydroxychloroquine (n=2).
Notable cases of neonatal COVID-19
A case reported by Han et al. [4] in Korea described viral load kinetics in multiple specimens of a neonate (the youngest) COVID-19 patient in Korea and her mother. This 27-day-old Korean female baby born at term had confirmed COVID-19 along with her mother and grandparents. The viral load of SARS-CoV-2 in the respiratory specimen of the neonate was the highest at the early stage of infection and gradually decreased with time. Notably, the viral load in the stool specimen remained high throughout her hospital stay, even after the respiratory specimens became negative. SARS-CoV-2 was also excreted at relatively low levels in the urine. These findings suggest that both stool and urine in neonates could be additional vehicles for viral transmission and that neonates with COVID-19 could have systemic complications [4].
Regarding SARS-CoV-2 infection in neonates, transmission routes other than droplets have not been confirmed. Whether and how SARS-CoV-2 can be transmitted from the mother to the fetus during pregnancy remains unclear. Vivanti et al. [24] demonstrated a neonatal case of SARS-CoV-2 through vertical or transplacental transmission. Such vertical transmission following maternal viremia, placental infection, and neonatal viremia was confirmed by comprehensive virological and pathological investigations. The viral load of SARS-CoV-2 in the placental tissue was much higher than that in any other specimen such as the maternal and neonatal blood. A placental histological examination revealed placental inflammation. Remarkably, the neonate presented with symptoms such as irritability, poor feeding, hypertonia, and opisthotonus. Encephalitic symptoms are very rare in neonates but commonly observed in adult patients [20]. Kirtsman et al. [18] and Kulkarni et al. [19] also demonstrated placental infection of SARS-CoV-2 in two neonates with COVID-19. According to the definition by Shah et al. [12], the case reported by Kirtsman et al. [18] represents a probable case of congenital SARS-CoV-2 infection in live born neonates, and the case reported by Vivanti et al. [24] represents a confirmed case of congenital SARS-CoV-2 infection in a live born neonate.
Vertical transmission of SARS-CoV-2
To date, it is believed that neonatal COVID-19 is mostly caused by horizontal transmission of SARS-CoV-2 through close contact with an infected person, usually his/her parent. The vertical transmission of SARS-CoV-2 can occur via transplacental transmission, intrapartum, or breastfeeding. Currently, the greatest concern in perinatal aspects of the COVID-19 pandemic is the possibility of vertical, especially transplacental, transmission of SARS-CoV-2. Vertical transmission has not been reported in either SARS or Middle East respiratory syndrome. Based on these findings, the possibility of vertical transmission of SARS-CoV-2 was thought to be very low in the early pandemic [46,47]. In a study detecting SARS-CoV-2 from different types of clinical specimens of 205 patients with COVID-19, only 1% (3 of 307) of blood specimens tested positive for RT-PCR, suggesting that placental and fetal seeding from maternal blood might be quite rare [48].
The first retrospective review of the possibility of vertical transmission of SARS-CoV-2 during pregnancy was reported in China. In 9 neonates born to 9 mothers with confirmed COVID-19 pneumonia, all samples collected from conception and the neonates, such as amniotic fluid, cord blood, neonatal throat swabs, and breastmilk tested negative for SARS-CoV-2 [49]. Another Chinese study described 2 neonatal cases born to mothers with COVID-19 during the third trimester. Serial RT-PCR assays also failed to detect SARS-CoV-2 in neonatal nasopharyngeal swabs, maternal serum, placental tissues, cord blood, amniotic fluid, vaginal swabs, and breastmilk [50].
However, several subsequent studies have suggested the possibility of vertical transmission. A cohort study described 33 neonates born to mothers with COVID-19, including 3 neonates with COVID-19 [27]. The nasopharyngeal and anal swabs in these 3 neonates tested positive for SARS-CoV-2 on days 2 and 4 of life. The authors suggested that SARS-CoV-2 in these neonates’ upper respiratory tracts and anuses is likely to have originated from their mothers, indicating that the vertical transmission of SARS-CoV-2 cannot be ruled out [27]. More recently, a neonate born to a mother with COVID-19 reportedly had elevated levels of SARS-CoV-2 immunoglobulin M (IgM), immunoglobulin G (IgG), and interleukin-6 2 hours after birth, with negative RT-PCR results for nasopharyngeal swabs taken from 2 hours to 16 days after birth. The RT-PCR results of the mother’s vaginal secretions and breastmilk were negative [51]. Elevated SARS-CoV-2 IgM and IgG levels in 2 neonates of mothers infected by COVID-19 with negative RT-PCR results for throat swabs and blood samples of neonates have also been reported [52]. However, the role of serology in the diagnosis of SARS-CoV-2 infection remains uncertain. Serologic findings in these 3 neonates are not evidence of a true congenital infection, but rather could represent an artifact [53].
Angiotensin-converting enzyme 2 (ACE2) is known to play essential roles in infection and transmission as a receptor of SARS-CoV-2 [54]. Based on the online available single-cell RNA sequencing database, ACE2 has very low expression in the placenta during the first trimester of pregnancy [55]. However, one study of fetal and postnatal mouse lung tissue revealed that the ACE2 level changes dynamically over time, peaking in neonatal mice at postnatal days 1–3 [54]. Together with 2 cases of congenital SARS-CoV-2 infection, these laboratory data suggest that transplacental transmission during late pregnancy could indeed be possible [18,24].
Laboratory and clinical findings to date can be summarized as shown below. First, direct evidence of the vertical transmission of SARS-CoV-2 is still lacking. Second, the possibility of vertical transmission during the third trimester of gestation cannot be ruled out. Third, the possible transmission of SARS-CoV-2 during early pregnancy and subsequent fetal outcomes remain unknown.
Transmission of SARS-CoV-2 via breastmilk
Although SARS-CoV-2 RNA has been detected in breastmilk in several studies, the majority of studies have not demonstrated SARS-CoV-2 in breastmilk [56-58]. In a recent observational cohort study, all 64 neonates breastfed by mothers with COVID-19 who undertook correct hygiene precautions tested negative for SARS-CoV-2 at 5–7 days and 14 days of life [59]. It remains unclear whether mothers with COVID-19 can transmit the virus via breastmilk. Current limited data suggest that it is unlikely [60].
Perinatal outcomes of pregnant women with COVID-19
In the early COVID-19 pandemic, there were no comparative studies that determined whether pregnancy might be a risk factor for severe COVID-19 [61]. One report from the WHO-China Joint Mission on COVID-19 concluded that pregnant women are not at a higher risk of developing severe disease due to COVID-19 than nonpregnant women [62]. However, subsequent studies revealed that pregnant women with COVID-19 might experience more severe disease [63,64].
From January 22 to June 7, 2020, the Centers for Disease Control and Prevention in the US analyzed the data of 91,412 women of reproductive age (15–44 years) with confirmed COVID-19 and for whom pregnancy status data were available [63]. Among them, 8,207 (9.0%) were pregnant. After adjusting for confounding factors, the authors found that pregnant women with COVID-19 were 5.4 times more likely to be hospitalized, 1.5 times more likely to be admitted to an ICU, and 1.7 times more likely to require mechanical ventilation than nonpregnant women with COVID-19. However, the risk of death was not significantly different between pregnant and nonpregnant women. This nationwide study shows that pregnant women and their families should be counseled about the potential risk of severe disease due to COVID-19 [63].
A retrospective case-control study in China reported outcomes of neonates born to mothers with COVID-19 [64]. In that study, from January 24 to February 29, 2020, neonatal outcomes of 36 pregnant women with COVID-19 pneumonia were compared with those of 121 pregnant women without COVID-19 admitted during the same period (2020 control group) and 121 women admitted between January 24 and February 11, 2019 (2019 control group). The results showed that rates of low birth weight (13.9%) and premature birth (22.2%) among neonates born to mothers with COVID-19 were significantly higher than those in the 2 control groups (2.5% and 5.4%, respectively) [64]. Although data on outcomes of patients with maternal and neonatal COVID-19 are still limited, pregnant women and their neonates might be at increased risk for developing severe COVID-19 and experiencing unfavorable birth outcomes.
Conclusion
The limited evidence available suggests a very low incidence and a relatively benign course of COVID-19 in neonates. Although a significant proportion of neonates in the present study required ventilator care, those with COVID-19 generally showed good outcomes without mortality. However, the sample sizes of studies of neonates with COVID-19 were too small to determine the risk of neonatal COVID-19. Despite the lack of direct evidence, there is growing concern about the risk of vertical transmission of SARS-CoV-2. Further research on the epidemiology, pathophysiology, and diagnosis of the vertical transmission of SARS-CoV-2 is urgently required.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The author is grateful for the support of the members of the Korean Society of Neonatology and Pediatric Infectious Diseases.