The global prevalence of Toxocara spp. in pediatrics: a systematic review and meta-analysis
Article information
Abstract
Background
Toxocariasis is a zoonotic parasitic disease caused by Toxocara canis and Toxocara cati in humans. Various types of T. canis are important.
Purpose
The current study aimed to investigate the prevalence of Toxocara spp. in pediatrics in the context of a systematic review and meta-analysis.
Methods
The MEDLINE (PubMed), Web of Sciences, Embase, Google Scholar, Scopus, and Cumulative Index of Nursing and Allied Health databases were searched to identify peer-reviewed studies published between January 2000 and December 2019 that report the prevalence of Toxocara spp. in pediatrics. The evaluation of articles based on the inclusion and exclusion criteria was performed by 2 researchers individually.
Results
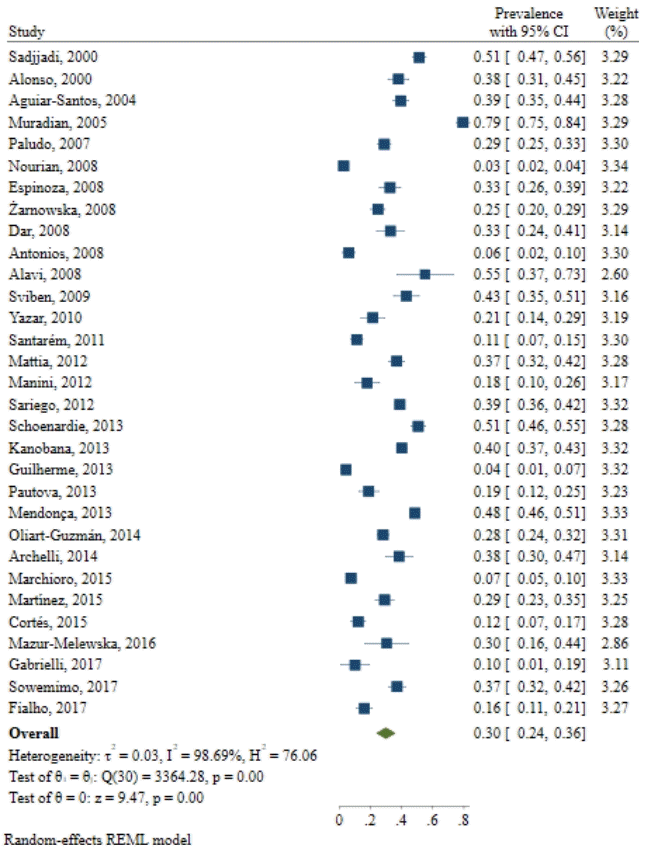
The results of 31 relevant studies indicated that the prevalence of Toxocara spp. was 3%–79% in 10,676 cases. The pooled estimate of global prevalence of Toxocara spp. in pediatrics was 30 (95% confidence interval, 22%–37%; I2=99.11%; P=0.00). The prevalence was higher in Asian populations than in European, American, and African populations.
Conclusion
Health policymakers should be more attentive to future research and approaches to Toxocara spp. and other zoonotic diseases to improve culture and identify socioeconomically important factors.
Key message
Is the global prevalence of toxocariasis high among children? The prevalence of toxocariasis is high in pediatric patients. Asian children are more susceptible to the disease than other children. Its virulence varies among different socioeconomic classes in various countries. Hand washing after soil contact, routine pet deworming, and appropriate disposal of pet feces in households with Asian pediatrics are needed to prevent toxocariasis.
Introduction
Toxocariasis or visceral migraine laryngeal syndrome is a zoonotic parasitic disease caused by Toxocara canis and Toxocara cati in humans. Various types of T. canis in particular can be important [1,2]. Each adult worm in the intestines of infected dogs and cats can release a large number of eggs daily through defecation. Toxocariasis is mostly transmitted to humans via water, food, and soil contaminated with eggs [2,3]. The eggs of this parasite are opened after entering the human digestive system. The larvae then pass through the intestinal mucosa to the bloodstream and diffuse into organs such as the liver, brain, and eye. Although the larva will be recognized and limited by the immune system forming granuloma, they can survive and persist in this form for up to 11 years [4].
The visceral larva migrans (VLM), ocular larvae (OLM), and overt toxocariasis are the most common types of larvae. The clinical symptoms of infection are nonspecific but can include neurological, ocular, pulmonary (asthmatic), dermatological, and rheumatoid arthritis [5]. The pathology of this parasite is variable, but it can induce peripheral eosinophilia (20%–40%) and fever of unknown origin. The prevalence of parasites varies among geographic regions at rates of 2%–90% [6-8].
Pediatrics are more susceptible to Toxocara infection since they are more likely to place a contaminated hand or even an egg into their mouth [9]. Seroepidemiological evaluations in different countries indicated a global distribution of toxocariasis. Considering the importance of the prevalence of this parasite in the population, this study aimed to evaluate the prevalence of toxocariasis in pediatric patients younger than 20 years of age based on a systematic review and meta-analysis of published studies.
Methods
This study was performed according to the MOOSE (MetaAnalyses of Observational Studies in Epidemiology) and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [10-12].
1. Search strategy
In this systematic review, we assessed all original research studies that were relevant to the topic by searching the MEDLINE (PubMed), Web of Sciences, Embase, Google Scholar, Scopus, and Cumulative Index of Nursing and Allied Health databases using keywords such as toxocariasis, Toxocara, Toxocara-antibodies, Pediatrics, Toxocara canis, and Toxocara cati. The search was limited to articles published between January 2000 and December 2019. The researchers searched these databases and manually searched the reference lists and gray literature. Duplicate entries were reviewed by considering the title of published papers, authors, year of publication, and specifications of the source types. In questionable records, the texts were compared. After abstract and title review, some of the articles were eliminated. The retrieved papers were evaluated by 2 researchers (YM, BA) based on the inclusion and exclusion criteria.
2. Eligibility criteria
For the current study, the inclusion criteria were publication in the English language and publication between January 2000 and December 2019. The study design and methodologies were cross-sectional. All other studies, such as reviews or meta-analyses, were excluded from the screening. Articles that were conducted on animals were also excluded.
We included all English observational studies published between January 2000 to December 2019 that assessed the prevalence of toxocariasis, therapeutic management, signs, and symptoms in pediatric patients with toxocariasis. Cross-sectional studies were also included. We excluded duplicate non–peer-reviewed, review, or meta-analysis articles and papers for which the abstracts and full texts were not available.
3. Data extraction
All of the included studies were listed by EndNote software (EndNote X7, Thomson Reuters, Toronto, ON, Canada) and subjected to review and data extraction by 3 independent authors. Two reviewers independently (BA and RGH) extracted the required data from the data contained in the identified articles using a uniform Excel sheet. Discrepancies in the extracted data were resolved through consensus. If agreement could not be reached, it was resolved by referral to a third investigator (YM). For the current study, the data extraction section contained the first author’s name, year of publication, study location, study type, sample size, age, positive population prevalence, and method of detecting the infection.
4. Risk of bias
The quality of all studies was assessed by 2 independent authors using the Modified Newcastle-Ottawa Scale for cross-sectional studies [13].
5. Statistical analysis
We used a random-effects model to generate a pooled prevalence presented as percentage and 95% confidence interval (CI) with Metaprop order. Interstudy heterogeneity was assessed using the I2 heterogeneity statistic reported as a percentage (%) to determine the extent of interstudy variation. A forest plot was used to present the meta-analysis results schematically. Egger test and a funnel plot were applied to evaluate the presence of publication bias. In addition, a subgroup analysis was performed to identify different sources of heterogeneity. The statistical analysis was performed using STATA 15.0 (Stata Corp., College Station, TX, USA), and statistical significance was set at P<0.05.
Results
The conducted search retrieved 2,395 studies. After duplicate removal and title and abstract screening, 276 relevant studies remained. The search and screening results are illustrated in Fig. 1. The assessment of 31 studies revealed that the prevalence of Toxocara was 3%–79% in 10,676 cases. Of the total number of children (10,676), 3,525 had Toxocara infection (Table 1). In addition, most of the conducted studies used enzymelinked immunosorbent assay (ELISA) to diagnose the infection. Furthermore, in most of the mentioned studies, the patients were younger than 12 years of age. The results from the different included studies are listed in Table 1.

Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram and search strategy results.
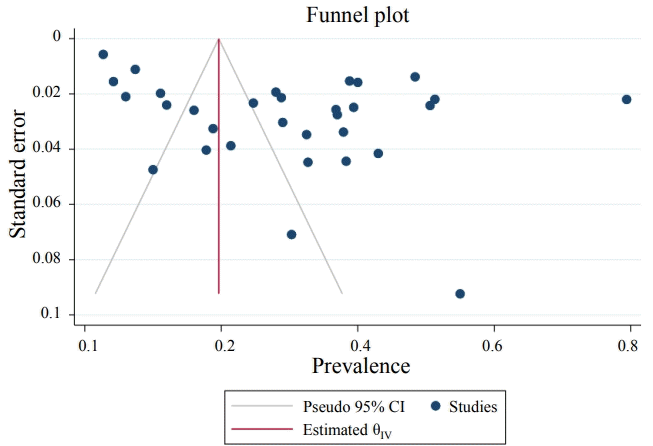
The results demonstrated that the pooled prevalence of Toxocara in pediatric patients worldwide was 30 (95% CI, 22%–37%; I2=99.11%; P=0.00) (Fig. 2), but since the CI of Egger test did not include zero, a significant bias occurred in the publication of the results (coefficient=11.58; T=4.39; P=0.001; 95% CI, 6.23–16.93). The funnel plot is shown in Fig. 3.
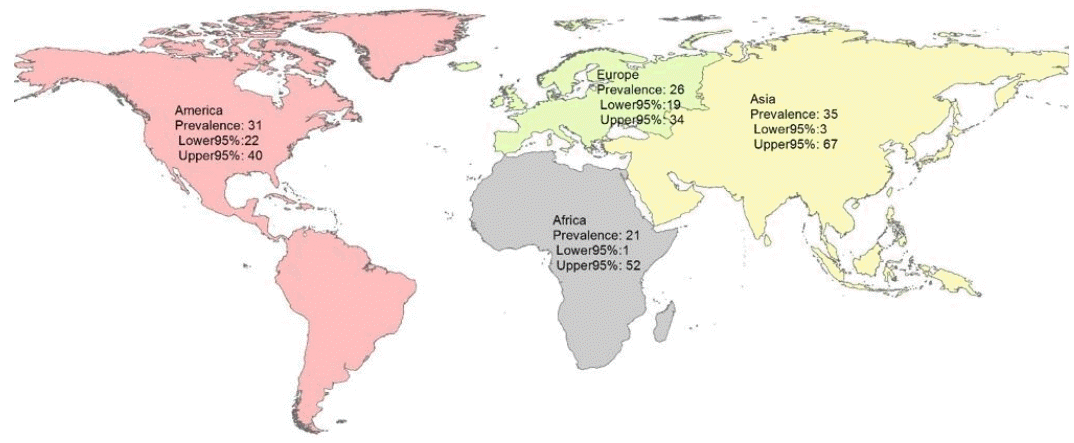
The subgroup analysis by continent showed that the pooled prevalence of Toxocara in pediatric patients in Asia, America, Africa, and Europe was 35% (95% CI, 3%–67%; I2=99.43%; P=0.00), 31% (95% CI, 22%–40%; I2=99.01%; P=0.00), 21% (95% CI, 1%–52%; I2=98.75%; P=0.00), and 26% (95% CI, 19%–34%; I2=85.94%; P=0.00), respectively (Table 2 and Fig. 4).
1. Meta-regression analysis
We used a meta-regression analysis to assess the effect of suspected variables such as year of study and sample size on heterogeneity. The results of the meta-regression analysis shown in Table 2 did not show any significant association between this variable and the prevalence of Toxocara spp. in pediatric patients (Table 3 and Fig. 5).

Meta-regression analysis to assess the effect of suspected variables on the pooled prevalence of Toxocara spp. in pediatric patients
Discussion
Toxocara is a worldwide worm and common intestinal parasite for cats and dogs that have the ability to infect humans [14]. Toxocara can be responsible for a variety of clinical manifestations [14]. The worm’s eggs can pass through the feces of animals and preserve infectivity for a long time in the soil [14]. Soil contamination with Toxoplasma is more frequent in humans [15]. It can be concluded that in some cases, closer contact with animals can increase the exposure risk for this parasite [9,16]. The current study aimed to systematically review the prevalence of Toxocara infection in pediatric patients. The results of 31 relevant studies indicated that the global prevalence of Toxocara in pediatric patients was 30 (95% CI, 22%–37%; I2=99.11%; P=0.00). The prevalence may vary among sample populations and geographical regions.
Oliart-Guzmán et al. [17] investigated the seroprevalence of Toxocara in households with children in Western Brazilian Amazon over 7 years. They concluded that the prevalence of infection in pediatric patients less than 5 years of age was 28%, 23.3%, and 13.9% in 2003, 2010, and 2011, respectively. Oliart-Guzmán et al. [17] suggested that water quality and the treatment of infected animals can be an appropriate strategy for preventing infection in these patients. Roldan et al. [18] investigated eosinophilia and other risk factors of Toxocara infection. They used 2 groups of seropositive and seronegative pediatrics. Their results showed that a dry cough and eosinophilia are significantly associated with Toxocara infection [17].
Serologic techniques are reliable methods that can detect larval antiantigens. The most commonly used serologic test is ELISA testing, which applies the secretion antigens of the parasite larvae [2]. Seroepidemiological evaluations in different countries have shown a global distribution of toxocariasis. The prevalence in American pediatrics is 4.6%–7.3% in the USA, 2.5% in Germany, and 19% in the Netherlands [19]. In Iran, the prevalence among school children was 25.6% in Shiraz and 31% in Ilam [20,21]. Musso et al. [22] reported the association of viral and bacterial central nervous system infections in pediatrics with Toxocara by evaluating the serum and cerebrospinal fluid of 381 patients. Toxocara immunoglobulin G (IgG) was present in 32% of meningitis and 34% of control group patients, a difference that was not statistically significant.
As mentioned above, Toxocara can have a variety of clinical manifestations [14]. Mazur-Melewska et al. [23] evaluated the pulmonary presentation of Toxocara in 119 positive patients who were 1–19 years of age. Their results suggested that high levels of eosinophilia and hyperimmunoglobulinemia E could be related to Toxocara infection. In addition, Pinelli et al. [24] assessed Toxocara infection in patients with suspected VLM and OLM from 1998 to 2009. Their results indicated a significantly decreased rate of infection in the Netherlands that has increased over time as the children grew up.
Epilepsy is a neurological disorder that leads to epileptic seizures [25,26]. Epilepsy can be idiopathic or due to trauma, hypoxia, or infection [27,28]. El-Tantawy et al. [29] assessed Toxocara infection in cryptogenic epilepsy patients. The etiology was unknown in these patients, but brain diseases are mostly suspected. Anti-Toxocara IgG was found in 48% of the cryptogenic epilepsy patients and 46 of the controls, but the difference was not statistically significant [29,30].
Sharghi et al. [31] investigated Toxocara infection as a risk factor for asthma in 95 patients aged 2–15 years and 229 controls. The result showed no statistically significant association between Toxocara and asthma, but Toxocara prevalence showed a significantly higher rate in Hispanic children of Puerto Rican descent. It can be concluded that this race is more susceptible to the disease. In addition, Silva et al. [32] reported the prevalence of Toxocara as a risk factor for atopia and asthma. They reported that the prevalence in northern Brazil is 63% in 791 patients and there is an association between Toxocara infection and serum IgE causing cross-reaction and atopia.
These results show a 35% prevalence of Toxocara infection in Asian pediatrics, a rate that is higher than that in other continents. We assume that the factors responsible for this variation likely represent differences in public health and sanitation status, cultural and social conditions, environmental hygiene, and climate [31,32]. This was a comprehensive meta-analysis of 31 valid publications evaluating 10,676 pediatrics on this topic. Most of the evaluated studies had an appropriate study population. The possibility that some patients were included in more than one report was low. The number of case reports in our assay was low, which leads to a lower risk of publication bias and increases the level of evidence of our findings. The limitations of this study are its high heterogeneity in the pooled estimate, differences in the diagnostic methods, and study population in primary studies.
According to this systematic review and meta-analysis, the global prevalence of Toxocara in pediatrics varies widely among geographical regions. The prevalence of Toxocara in Asian pediatrics was higher than those in other continents, which shows that in this country and in other continents, health policymakers should be more focused on future research and approaches related to Toxocara and other zoonotic diseases, improve the culture, and identify socioeconomically important factors.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.