Perinatal outcome and possible vertical transmission of coronavirus disease 2019: experience from North India
Article information
Abstract
Background
The consequences of severe acute respiratory syndrome corona virus 2 on mother and fetus remain unknown due to a lack of robust evidence from prospective studies.
Purpose
This study evaluated the effect of coronavirus disease 2019 (COVID-19) on neonatal outcomes and the scope of vertical transmission.
Methods
This ambispective observational study enrolled pregnant women with COVID-19 in North India from April 1 to August 31, 2020 to evaluate neonatal outcomes and the risk of vertical transmission.
Results
A total of 44 neonates born to 41 COVID-19–positive mothers were evaluated. Among them, 28 patients (68.3%) (2 sets of twins) were delivered within 7 days of testing positive for COVID-19, 23 patients (56%) (2 sets of twins) were delivered by cesarean section; 13 newborns (29.5%) had low birth weight; 7 (15.9%) were preterm; and 6 (13.6%) required neonatal intensive care unit admission, reflecting an increased incidence of cesarean delivery and low birth weight but zero neonatal mortality. Samples of cord blood, placental membrane, vaginal fluid, amniotic fluid, peritoneal fluid (in case of cesarean section), and breast milk for COVID-19 reverse transcription-polymerase chain reaction tested negative in 22 prospective delivery cases. Nasopharyngeal swabs of 2 newborns tested positive for COVID-19: one at 24 hours and the other on day 4 of life. In the former case, biological samples were not collected as the mother was asymptomatic and her COVID-19 report was available postdelivery; hence, the source of infection remained inconclusive. In the latter case, all samples tested negative, ruling out the possibility of vertical transmission. All neonates remained asymptomatic on follow-up.
Conclusion
COVID-19 does not have direct adverse effects on the fetus per se. The possibility of vertical transmission is almost negligible, although results from larger trials are required to confirm our findings.
Key message
Question: Is there any risk of vertical transmission of coronavirus disease 2019 (COVID-19), and what is its neonatal profile?
Finding: Biological samples for vertical transmission were negative in all deliveries; however, 2 neonates tested positive for nasopharyngeal COVID-19 reverse transcription-polymerase chain reaction. No significant neonatal morbidity was observed.
Meaning: COVID-19 does not increase adverse neonatal outcomes and shows a negligible risk of vertical transmission; however, horizontal transmission cannot be underestimated.
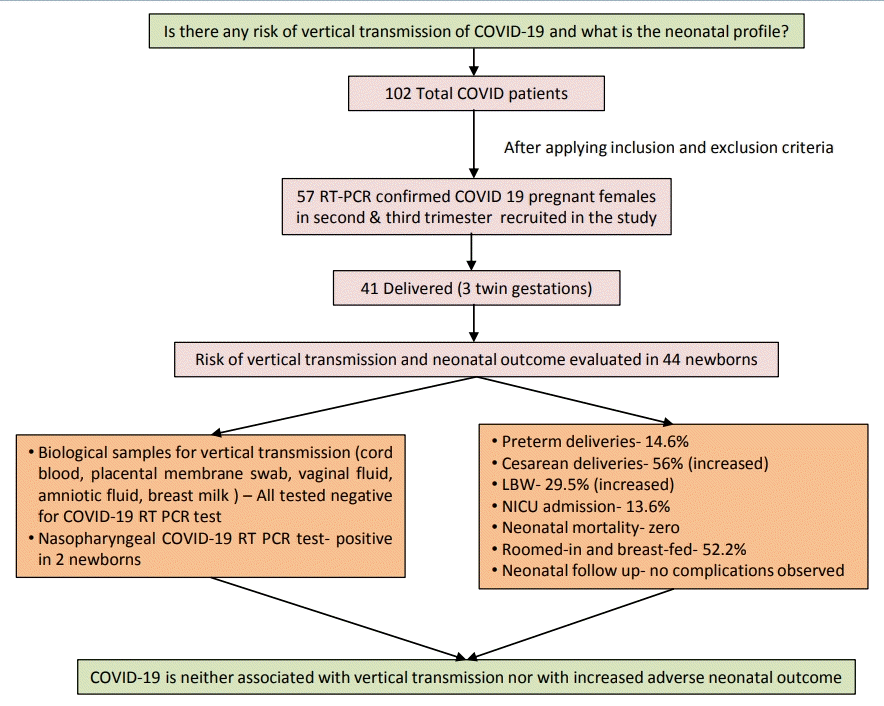
Graphical abstract. Coronavirus disease 2019 (COVID-19) vertical transmission risk and neonatal outcomes. RT-PCR, reverse transcription-polymerase chain reaction; LBW, low birth weight; NICU, neonatal intensive care unit.
Introduction
With evolving coronavirus disease 2019 (COVID-19) pandemic and the new data emerging, though minimal, the possibility of vertical transmission cannot be denied. Further, the impact of severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) on perinatal outcome is also uncertain. Most studies are retrospective and included third trimester patients. However, long-term prospective studies evaluating the effect on second and third trimester are lacking. Few studies reported increased incidence of cesarean, preterm labor, low birth weight, and NICU admission [1,2]. Some studies raised concerns regarding the possibility of vertical transmission while others contradict the same [3-6]. Keeping in view the scarcity of evidence to standardize the protocols for neonatal evaluation and management due to insufficient good quality data, we undertook this ambispective observational study to answer various queries.
Objectives of this study were to evaluate the scope of vertical transmission, if any, and to evaluate the effect of COVID-19 on neonatal outcome.
Methods
1. Study population
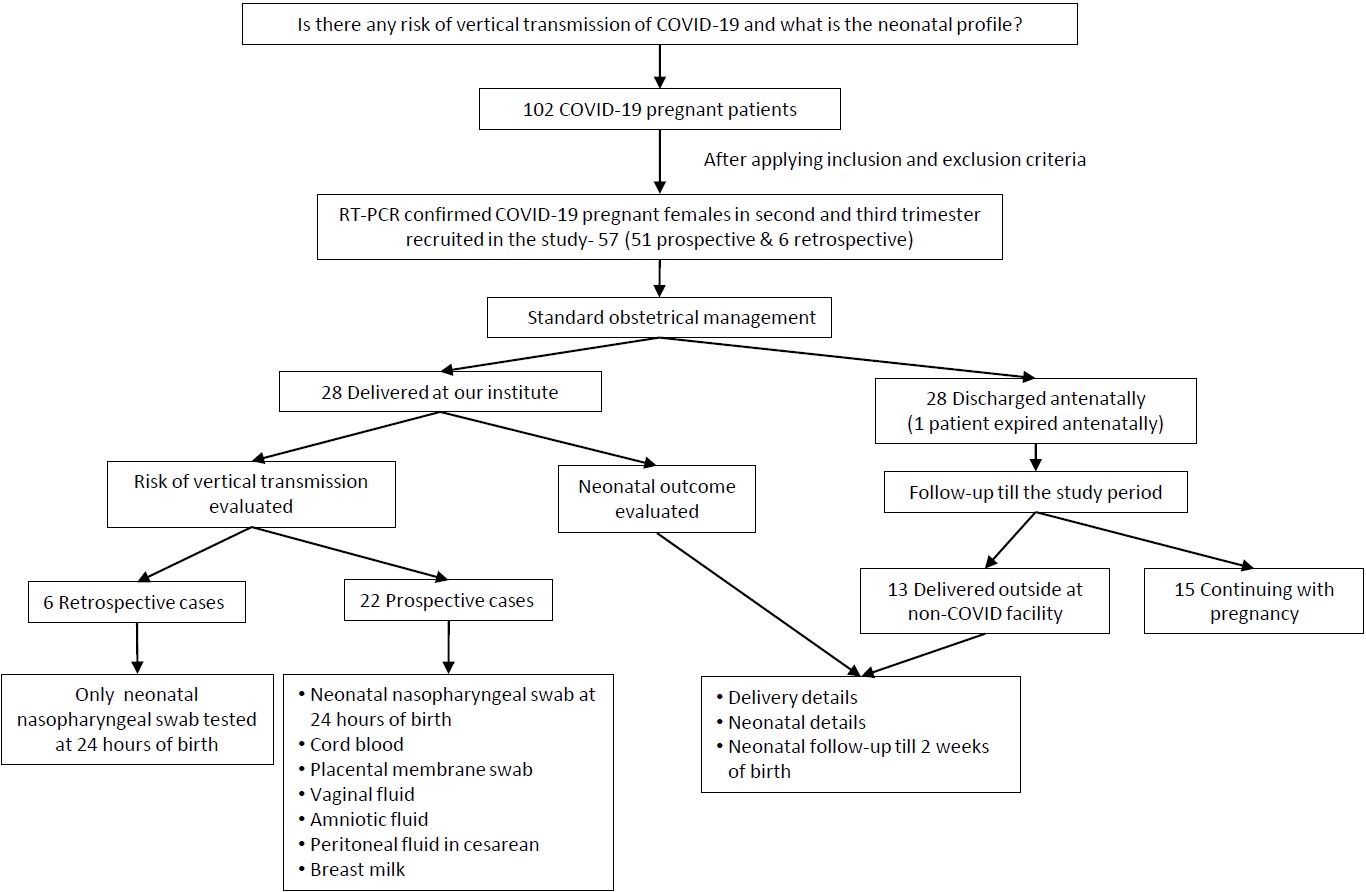
In this ambispective (including retrospective and prospective cases) observational study, approved by the institutional ethics committee (GIMS/IEC/HR/2020/13), we included real-time reverse transcription-polymerase chain reaction (RT-PCR) confirmed COVID-19 pregnant patients admitted at our institute from 1st April 2020 to 31st August 2020 after obtaining their written informed consent. Universal screening of pregnant patients admitted at our institute (whether symptomatic or asymptomatic) was being practiced as per regional guidelines. The inclusion criteria were symptomatic/asymptomatic RT-PCR confirmed COVID-19 pregnant patients in second and third trimester. The exclusion criteria were patients without RT-PCR confirmed COVID-19 disease, patients in first trimester, and postpartum patients.
2. Study protocol
The recruited patients were managed as per the national COVID-19 guidelines for the pregnant patients [7]. The details of patients who got delivered till 31st August 2020 were evaluated with respect to neonatal outcome and scope of vertical transmission. To assess vertical transmission risk we considered only those COVID-19 positive participants who delivered within the facility. For retrospective patients who delivered at our institute, only nasopharyngeal RT-PCR test of the neonate was done at 24 hours of birth to evaluate the scope of vertical transmission. For prospective study participants who delivered at our institute, apart from nasopharyngeal swab of neonate, we sent the samples of cord blood, placental membrane swab, vaginal fluid, amniotic fluid, peritoneal fluid (in case of cesarean section), and breast milk for COVID-19. All samples were processed for SARS-CoV-2 using RT-PCR (Quantstudio 5). The kit used was A*STAR Fortitude Kit 2.0 (Accelerate Technologies Pte Ltd [DxD Hub], Singapore) along with positive and negative controls. The diagnosis and management of newborns with or at risk of COVID-19 were in accordance with standard national guidelines [7,8]. For healthy neonates, parents were counseled and given the option of either rooming in and breastfeeding or getting temporarily separated from mother. For neonates who were roomed in, the nasophayngeal swab was again tested for COVID-19 at the time of discharge. The neonates with positive COVID-19 RT-PCR report were referred to pediatrics super specialty institute for further management as per our protocol. Routine immunization was implemented and all neonates were followed till discharge or till 2 weeks after delivery (telephonically) whichever was later and were assessed for respiratory, gastrointestinal, and other symptoms, if any.
The recruited patients who were discharged antenatally were followed for delivery till the period of study. The records of those who delivered outside at non- COVID facility were retrieved for delivery details, neonatal outcome, and any neonatal complication during follow-up period (Fig. 1).
3. Statistical analysis
For statistical analysis, continuous variables were described as mean (standard deviation) or median (interquartile range). Categorical variables were represented as frequencies with proportions. We used software EpiInfo 7.2 (CDC, Atlanta, GA, USA) for analysis of data.
Results
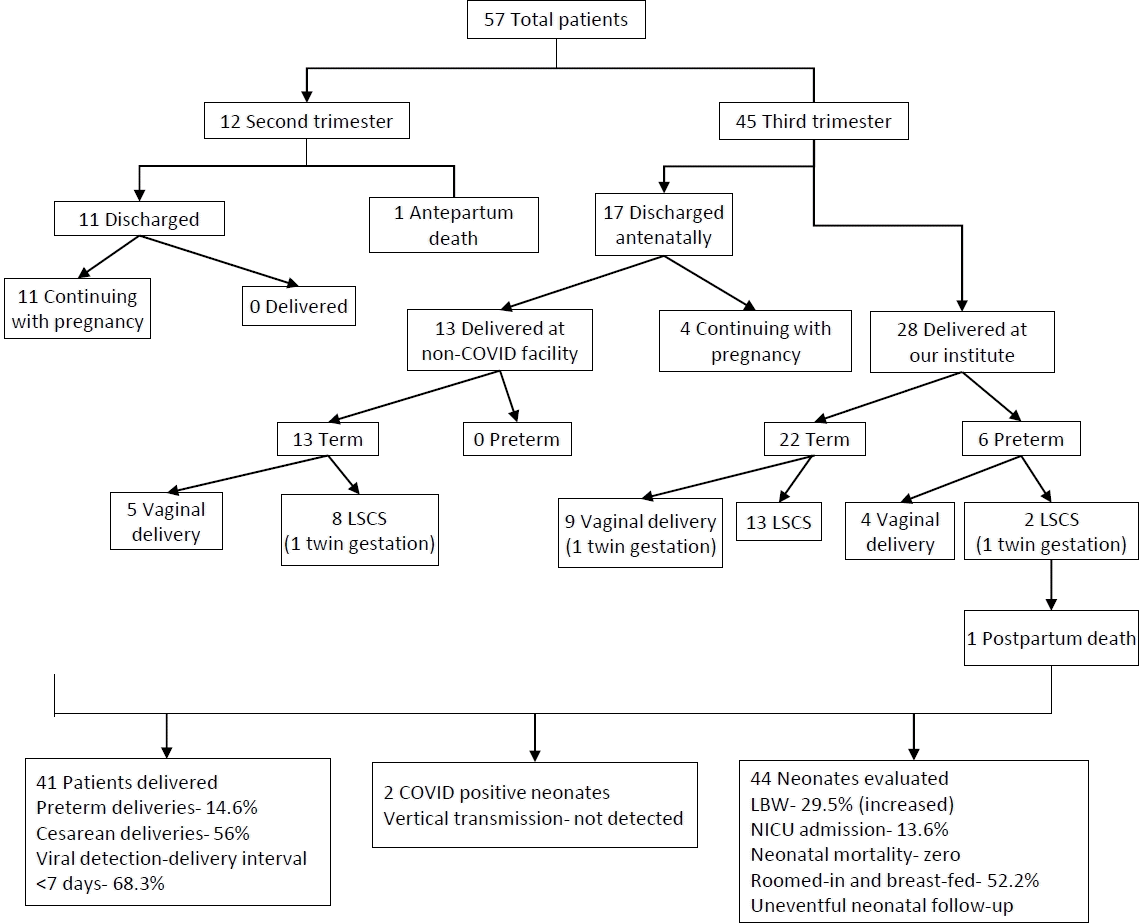
We reported the outcome of 44 neonates born to 41 COVID-19 infected females (including 3 twin gestations) from 1st April 2020 to 31st August 2020 at our institute. Out of 102 pregnant COVID patients we included 57 eligible pregnant patients, 12 second trimester, and 45 third trimester with RT-PCR confirmed COVID-19 infection in the study with 6 cases being retrospective (Fig. 2). Among them, 28 patients (including 2 twin gestations) delivered at our institute with 1 maternal death in postpartum period due to MODS (multiorgan dysfunction syndrome) with probable cause of death as COVID related acute liver and kidney injury or partial HELLP (hemolysis, elevated liver enzymes, and low platelet) syndrome. Also, 1 patient in second trimester with COVID pneumonia with acute respiratory distress syndrome expired antenatally. The rest 28 patients (11 in second trimester and 17 in third trimester) were discharged after treatment and followed up. Of 17 third trimester patients, 13 were delivered at non-COVID facility (including 1 twin gestations) and their delivery details and neonatal outcomes were noted while rest 4 patients in third trimester along with 11 patients in second trimester (total 15) were still continuing with the pregnancy (Fig. 2).
Outcomes of recruited pregnant COVID-19–positive patients. COVID-19, coronavirus disease 2019; LSCS, lower segment cesarean section; LBW, low birth weight; NICU, neonatal intensive care unit.
Out of 41 females with COVID-19 infection, over two-thirds (28 [68.3%]) delivered within 7 days from being tested positive (viral detection- delivery interval); 23 (56%) underwent cesarean section with fetal distress and previous cesarean section being most common indications. Among the patients who delivered at our institute 22 (78.6%) delivered at term while 6 (21.4%) delivered before 37 completed weeks. All deliveries outside our institute at non-COVID facility were term (Table 1, Fig. 2).
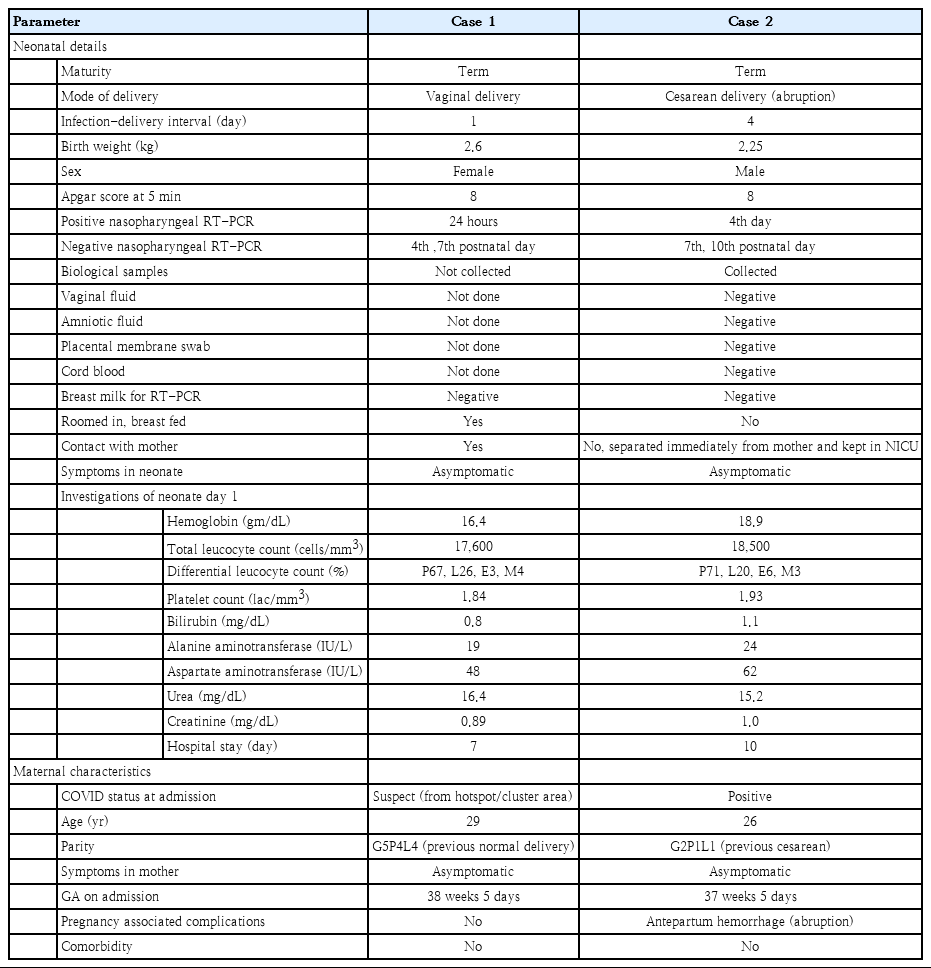
Among 28 patients delivered at our institute, for 22 prospective study participants, we sent the biological samples – cord blood, placental membrane swab, vaginal fluid swab, amniotic fluid, peritoneal fluid (in 10 cases of cesarean section), and breast milk for COVID-19 RT-PCR to evaluate the scope of vertical or horizontal transmission and all tested negative (Table 1). Among all neonates (n=30) born at our institute, the nasopharyngeal swabs of 2 neonates (6.6%) reported positive for SARS-CoV-2 by RT-PCR; both remained asymptomatic and their clinical profile is described in Table 2.
Out of 44 newborns, low birth weight was reported among 13 neonates (29.5%); 7 neonates (15.9%) were preterm; 24 (54.5 %) were males; 5 (11.4%) had Apgar score <7 at 5 minutes and 6 (13.6%) required neonatal intensive care unit (NICU) admission mainly for prematurity and birth asphyxia. There was no stillbirth or neonatal death. Majority had near normal biochemical profile and no severe neonatal complications.
A total of 23 (52.2%) were roomed in and breast fed. All neonates were followed till 2 weeks without any reported untoward outcome. The detailed outcome of neonates delivered at our institute and those delivered after being discharged at non-COVID facility have been tabulated in Table 1.
Discussion
To our knowledge, this is the first ambispective study from North India with a significant cohort of prospective study participants describing the outcome of neonates born to the COVID-19 positive mothers and the risk of perinatal transmission of COVID-19. Most of the studies are retrospective barring few [1,4,9,10].
We report 14.6% preterm delivery rate in our study. Earlier studies have reported very high incidence ranging from 36.4% to 60% [1,11,12]. Smith et al. [2] in the systematic review reported 63.8% preterm birth and attributed that to viremia. In another review Melo et al. [13] observed only 16.6% incidence of preterm births, endorsed by other studies also and our study findings corroborates with the same [4,14]. The possible explanation may be high prevalence of asymptomatic or mild cases of COVID-19 in pregnancy where viremia was insufficient to trigger parturition cascade. While evaluating early preterm births we found that 3.6 % of newborns were delivered below 32 weeks. Knight et al. [10] reported the incidence at same gestational age as 8.6%.
Mean viral detection-delivery interval in our study was 7.97±8.45 days which was much higher than that reported by Smith et al. [2] (4.3±3.08 days). Two-thirds patients (68.3%) in our study delivered within 7 days of being tested positive for COVID-19, out of these patients 78.6% were term deliveries. This is a noteworthy point that it was gestational age and not COVID-19 per se which resulted in these births.
Yan et al. [3] included 99 COVID-19 pregnant women in their study and found 85.9% cesarean rate with COVID-19 pneumonia as the most common indication. Other studies too reported 78.7% to 100% of cesarean deliveries, mostly iatrogenic [1,11,12,14]. Current guidelines advocate cesarean section for obstetrical indications only since one mode of delivery has not shown any benefit over the other as far as the neonatal COVID status is concerned [7,15,16]; hence the cesarean rate is decreasing which in our study was 56% and Salvatore et al. [9] recorded even lesser rate of 44%.
Neonates can get infected via vertical transmission or through contact. The vertical transmission broadly includes transmission of infection in-utero, during delivery or during breastfeeding and to detect its possibility various biological samples need to be tested. Centers for Disease Control and Prevention reported 2.6% incidence of neonatal COVID-19 [17]. Knight et al. [10] in a prospective study including 262 neonates born to COVID-19 mothers reported 4.6% incidence, and in our study, we a report 6.6% incidence of neonatal COVID-19. In their study, they used only nasopharyngeal swab assess the risk of vertical transmission like most other studies. We want to highlight that timing of nasopharyngeal swab testing of neonates in different studies varied – at birth, within 24 hours, at 24 hours, or at 48 hours; attributed to its initial nonstandardization in different guidelines [1,9,10,14]. In retrospective study by Yan et al. [3] on 100 neonates, nasopharyngeal swab was taken in 86 neonates with additional samples of amniotic fluid and cord blood tested in 10 cases, vaginal fluid in 6 cases, and breast milk in 12 cases only. By testing only neonatal nasopharyngeal sample, one cannot confirm or exclude vertical transmission, just like case 1 (retrospective case) in our study. So, to confirm vertical transmission, we went one step ahead and got all other biological samples tested in 22 prospective deliveries – cord blood, placental membrane swab, vaginal fluid, amniotic fluid, peritoneal fluid (in 10 cesarean section), and breast milk (n=23) and all samples tested negative.
We proceeded with the study as sporadic case reports and case series tested one or the other biological sample positive warranting further research to assess the risk of vertical transmission. Penfield et al. [18] described 3 positive placental/membrane swabs for COVID-19 by RT-PCR test although all the neonates tested negative. It has been proposed that since angiotensin- converting enzyme 2 receptors are expressed widely on the placenta, it can get infected by COVID-19 virus. Costa et al. [19] apart from placenta also described positive cord blood and breast milk samples without neonatal positivity. Kirtsman et al. [20] reported a case of COVID-19 positive neonate born via cesarean section where vaginal fluid along with placenta, and breast milk tested positive for SARS-CoV-2. However, with emerging evidence regarding lack of angiotensin-converting enzyme 2 (ACE2) receptors in vaginal epithelium, the possibility of virus being present there is negligible [3,21]. We also tested all our vaginal fluid samples negative for COVID-19. Zamaniyan et al. [21] reported a COVID positive preterm female baby delivered via cesarean section with positive amniotic fluid sample. IgM antibodies were detected in 2 neonates by Zeng et al. [22], and in one neonate by Dong et al. [23] though their nasopharyngeal swabs were negative. Passive transfer across the placenta from mother to fetus can be the explanation for IgG antibodies but not for IgM antibodies as they do not cross placenta and take at least 3–7 days for synthesis; hence presence at birth indicates neonatal immune response to in-utero infection. The possibility of contracting the infection from peritoneal fluid was also raised [24]. To address the above-reported findings in the literature we undertook testing of all biological samples which reported negative. Hence, we strongly negate the risk of vertical transmission in COVID-19 due to availability of robust evidence with us.
Various studies reported 9%–22.2% incidence of low birth weight [5,9,14], 1%–22.2% incidence of birth asphyxia [1,3,5,14], 5.2%–47% NICU admission [1,3,10,25] and 0%–1% neonatal deaths in COVID-19 pregnancies [3,9,10] with 1.1%–10% stillbirths [10,12]. Trocado et al. [26] in a review including 8 studies involving 95 COVID infected pregnant women and 51 neonates reported 20% low birth weight, no cases of severe neonatal asphyxia and 2% neonatal death though it was not related to the viral infection. In another review by Juan et al. [27], 221 neonates from consecutive case series, there were 3.6% low birth weight babies, 0.4% cases of neonatal asphyxia, a third babies were admitted in NICU, and 0.4% neonatal deaths.
In our study 29.2% had low birth weight, 88.6% had Apgar score more than 7 at 5 minutes, 11.4% neonates had birth asphyxia, 13.6% NICU admission with no neonatal death. This is in accordance with the prospective study conducted by Salvatore et al. [9] who recorded 13% low birth weight, 15% NICU admission, and no neonatal mortality suggesting that COVID-19 does not have increased adverse perinatal outcome. Majority of neonates in various studies, even those who were COVID positive remained asymptomatic with near normal biochemical profile and no severe neonatal complications. We would like to highlight that in most studies the NICU admission was done to isolate and observe the neonates born to COVID-19 positive mothers rather than any absolute indication and there was no associated increased neonatal mortality. Reduced distribution of ACE2 receptors, reduced cytokine storm due to immature immune system and routine immunization are the possible explanations for the mild COVID disease in newborns.
In the initial phase of pandemic, various guidelines advocated immediate separation of neonates from mothers after delivery considering COVID-19 highly infectious. Once it became clear with the emerging evidence that the breast milk does not contain viable virus and the risk of neonatal COVID-19 infection is similar in those separated from mothers and those being roomed in with infection prevention and control practices in place, the guidelines were revised. Although World Health Organization [28], American Academy of Pediatrics [29], Royal College of Obstetricians & Gynaecologists [15], and Federation of Obstetric and Gynaecological Societies of India [7] endorse breastfeeding and rooming in while keeping mother-neonate dyad in separate postpartum unit, this is not being followed because of dearth of evidence and resources. The only good quality prospective study promoting rooming in and breastfeeding which we could find was from New York by Salvatore et al. [9] where out of 82 neonates, 83% neonates were roomed in, 78% were breast fed and no newborn was reported to have COVID-19, thereby concluding that if infection control practices are religiously followed, the risk of perinatal transmission is very low. In other prospective studies by Antoun et al. [1] and Knight et al. [10], nothing is mentioned about rooming in and breastfeeding of the neonates. In our study, even after counseling the parents regarding the benefits of breastfeeding and the minimal risk of neonate getting infected via contact, only 30% babies delivered at our facility were roomed in and breastfed. Although the mothers followed preventive measures yet one neonate tested positive for COVID-19. Considering the ethical issues as well as infrastructure and manpower crunch in developing countries, we suggest that till the time robust evidence is available from the developed world, although we should be encouraging rooming in and breast feeding yet the autonomy of parents in decision making to be respected.
We followed up the neonates after birth till 2 weeks or discharge whichever was later. Follow-up of neonates is essential to detect late infection or any other complication. In most of the studies, this neonatal follow-up was lacking. In the prospective study by Salvatore et al. [9], the neonates were followed up till 1 month of age and all had normal growth without any significant complaints. They also got nasopharyngeal swabs of roomed in neonates tested for COVID-19 at birth, 7 days and at 14 days of life during follow-up and all reported negative demonstrating no vertical or horizontal transmission of COVID-19. In our study, too all the neonates remained asymptomatic on follow-up as far as COVID symptoms were concerned, again negating unfavorable outcome of neonates born to COVID positive mothers.
The strengths of our study include prospective cohort with good sample size; testing of all the biological samples for vertical transmission and follow-up of neonates for the COVID-19 symptoms. There are certain limitations too; comparison with non-COVID-19 pregnant patients would have enlightened the difference in neonatal outcome, if any, which was not possible in our setting as ours was a dedicated tertiary COVID-19 center. Also, antibody testing may have added to the evidence related to vertical transmission though the possibility is trivial Further, the findings in our study may not be generalized as it is a single center study.
Our study concludes that COVID-19 is not associated with increased adverse neonatal outcome; even most of the infected neonates remain asymptomatic. Further, as per evidence generated, the possibility of vertical transmission of COVID-19 is extremely low. Larger prospective studies with neonatal follow-up are needed to provide robust evidence related to the scope of perinatal transmission if any.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We would like to thank all the residents, nursing staff, and the laboratory staff involved in the management of these motherneonate dyads.