Update on infantile hemangioma
Article information
Abstract
The International Society for the Study of Vascular Anomalies classifies vascular anomalies into vascular tumors and vascular malformations. Vascular tumors are neoplasms of endothelial cells, among which infantile hemangiomas (IHs) are the most common, occurring in 5%–10% of infants. Glucose transporter-1 protein expression in IHs differs from that of other vascular tumors or vascular malformations. IHs are not present at birth but are usually diagnosed at 1 week to 1 month of age, rapidly proliferate between 1 and 3 months of age, mostly complete proliferation by 5 months of age, and then slowly involute to the adipose or fibrous tissue. Approximately 10% of IH cases require early treatment. The 2019 American Academy of Pediatrics clinical practice guideline for the management of IHs recommends that primary care clinicians frequently monitor infants with IHs, educate the parents about the clinical course, and refer infants with high-risk IH to IH specialists ideally at 1 month of age. High-risk IHs include those with life-threatening complications, functional impairment, ulceration, associated structural anomalies, or disfigurement. In Korea, IHs are usually treated by pediatric hematology-oncologists with the cooperation of pediatric cardiologists, radiologists, dermatologists, and plastic surgeons. Oral propranolol, a nonselective beta-adrenergic antagonist, is the first-line treatment for IHs at a dosage of 2–3 mg/kg/day divided into 2 daily doses maintained for at least 6 months and often continuing until 12 months of age. Topical timolol maleate solution, a topical nonselective beta-blocker, may be used for small superficial type IHs at a dosage of 1–2 drops of 0.5% gel-forming ophthalmic solution applied twice daily. Pulse-dye laser therapy or surgery is useful for the treatment of residual skin changes after IH involution.
Key message
· Infantile hemangiomas (IHs) are the most common benign vascular tumors, occurring in 5%–10% of infants.
· IHs are characteristically not present at birth but are usually diagnosed at 1–4 weeks of age, rapidly proliferate until 5 months of age, and then spontaneously involute.
· High-risk IHs (10%) require early treatment from 1 month of age.
· Oral propranolol, a nonselective beta-blocker, is the first-line treatment for IHs.
Graphical abstract
Introduction
During the past decade, there have been great advances in the biology, classification, terminology, diagnosis, and treatment of vascular anomalies (VAs). The International Society for the Study of Vascular Anomalies (ISSVA; https://www.issva.org) published the ISSVA classification for VA in 2014 and revised version in 2018, which classifies VA into 2 large categories of vascular tumors and vascular malformations [1-7]. Vascular tumors are defined as vascular neoplasms caused by the proliferation and hyperplasia of abnormal endothelial and other vascular cells, while vascular malformations are defined as congenital developmental disorders consisting of capillary, lymphatic, venous, and arterial vessel formation [2-10]. Vascular tumors feature increased endothelial turnover (mitosis), while vascular malformations do not [3].
Infantile hemangiomas (IHs) are the most common vascular tumor and the most common benign tumor of infancy, developing in 5%–10% of infants [5,8-13]. Pathologically, IHs are glucose transporter-1 protein (GLUT-1)–positive, the expression of which distinguishes them from other vascular tumors or vascular malformations [5,7,8,10,11]. IHs are clinically characterized by a proliferative phase followed by an involutive phase [10,14,15]. Because of the spontaneous involution, most IHs do not require treatment, but about 10% of cases require early treatment due to size, location, and complications [9,11-13]. Since 2015, IH management guidelines have been published in Europe, America, Australia, and Japan [12,13,16-18]. The American Academy of Pediatrics (AAP) published its first clinical practice guideline (CPG) for the management of IHs in 2019, which recommends that pediatricians and other primary care clinicians frequently monitor infants with IHs during the first few weeks and months of life to screen for growth and complications, educate the parents about the clinical course, and refer infants with potentially problematic IHs to IH specialists as early as 1 month of age [11]. Potentially problematic or high-risk IHs include those with life-threatening complications, functional impairment, ulceration, associated structural anomalies, and disfigurement [11-13]. Primary care clinicians are also recommended the referral to IH specialists when the IH diagnosis is uncertain and requires differentiation from other vascular tumors or vascular malformations [9,11,13]. Imaging modalities such as ultrasonography (US) with Doppler, magnetic resonance imaging (MRI), and/or magnetic resonance angiography (MRA) may be used to diagnose IHs and monitor their treatment response [9,11-13]. In Korea, IHs are usually treated by pediatric hematology-oncologists with the cooperation of pediatric cardiologists, radiologists, dermatologists, and plastic surgeons [19-22].
The AAP CPG for the management of IHs recommends oral propranolol, an oral nonselective beta-adrenergic antagonist, as the present gold standard treatment for high-risk IHs at a dosage of 2–3 mg/kg/day divided into 2 daily doses maintained for at least 6 months [11]. This AAP CPG also approves topical timolol maleate, a topical nonselective beta-blocker, for the treatment of some small, thin, superficial IHs at a dose of 1–2 drops of 0.5% gel-forming ophthalmic solution applied twice daily [11]. Pulse-dye laser therapy (PDL) or surgery are recommended for the treatment of residual skin changes after IH involution but may be used earlier to treat selected cases of IHs [9,11,12,16].
ISSVA classification of VAs
The ISSVA classification of VA better explains the terminologies, biology, and clinical characteristics of VA, including vascular tumors and vascular malformations, and is now considered the clinical gold standard for the care of patients with VA [1]. The 2018 ISSVA classification for VA is summarized in Table 1 [1]. Vascular tumors are classified as benign, locally aggressive or borderline, or malignant [1,5]. Benign vascular tumors include IHs, congenital hemangiomas (CHs), tufted hemangioma, spindle-cell hemangioma, epithelioid hemangioma, and pyogenic granuloma (also known as lobular capillary hemangioma). CHs are further classified as rapidly involuting CH (RICH), noninvoluting CH (NICH), and partially involuting CH (PICH) depending on their clinical progression. Locally aggressive or borderline vascular tumors include Kaposiform hemangioendothelioma, other hemangioendotheliomas, and Kaposi sarcoma. Malignant vascular tumors include angiosarcoma and epithelioid hemangioendothelioma. Vascular malformations are classified as simple or combined vascular malformations, of the major vessels, and vascular malformations associated with other anomalies [1,2]. Simple vascular malformations are subgrouped as slow blood flow (capillary, lymphatic, venous malformations [VMs]) or fast blood flow (arteriovenous malformations [AVMs], arteriovenous fistula) depending on the blood flow [1,2].
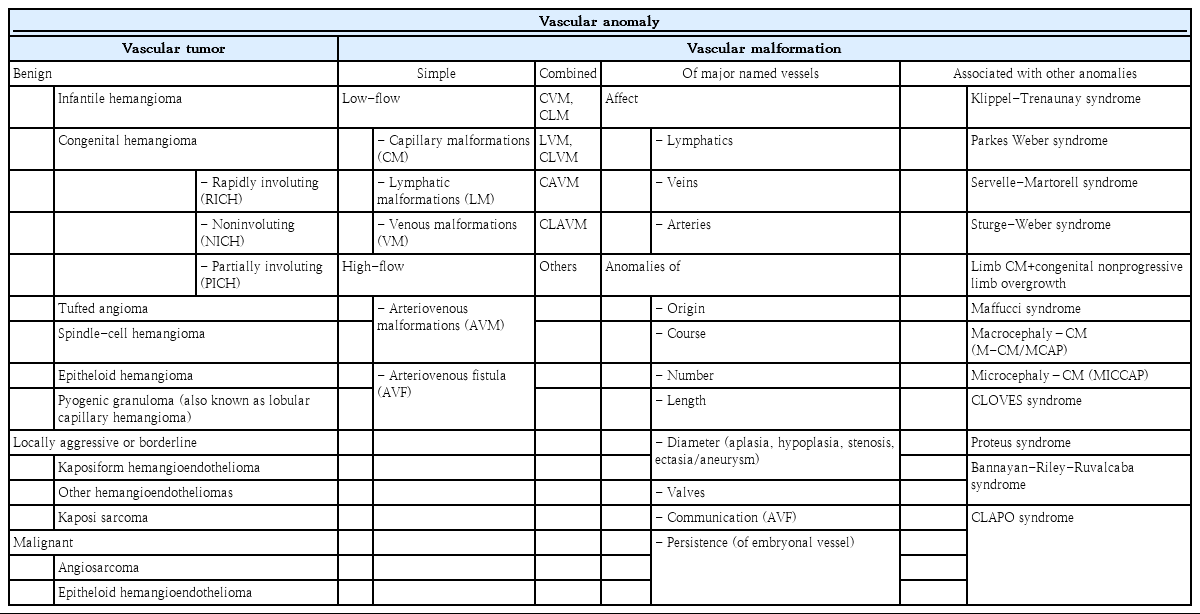
The 2018 International Society for the Study of Vascular Anomalies (ISSVA) classification for vascular anomalies
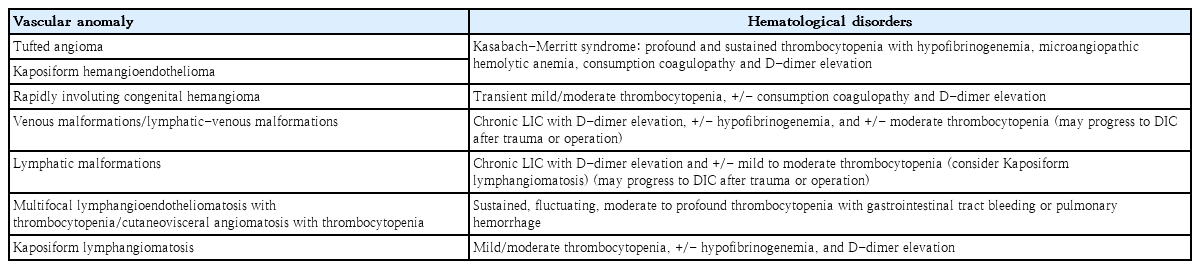
VA can be associated with hematologic disorders such as thrombocytopenia and/or consumption coagulopathy, some of which cause life-threatening Kasabach-Merritt phenomenon (KMP; the combination of rapidly enlarging hemangioma, thrombocytopenia, microangiopathic hemolytic anemia, and acute or chronic consumption coagulopathy with D-dimer elevation) (Table 2) [1]. Tufted angioma or Kaposiform hemangioendothelioma, rather than IHs, cause KMP and require emergency referral [5,8,23-25].
Infantile hemangiomas
IH is a benign vascular tumor with abnormally increased proliferation of vascular endothelial cells and aberrant blood vessel architecture [5,11-13,16,26].
Epidemiology of IH
IH is the most common vascular tumor and most common benign tumor of infancy, developing in 5% of infants and in up to 10% of Caucasian infants [5,11-13,16,26]. IHs develop more commonly in cases of female infants (female to male ratio, 1.4:1 to 3:1), twins, Caucasians, premature infants, low birth weight infants (up to 30% of infants with a birth weight < 1 kg), multiple pregnancies, increased maternal age, preeclampsia, placental anomalies (placenta previa, placental abruption, abnormal insertion of the umbilical cord), and a family history of IH in a first-degree relative [9,11-13,16,27].
Pathogenesis of IH
The pathogenesis of IH has not yet been completely defined, and current studies support several hypotheses. The first hypothesis is the somatic mutation of hemangioma stem cells (HemSC) and upregulation of angiogenic vascular endothelial growth factor receptor (VEGFR) signaling. This theory suggests that IH is derived from CD34+/CD133+ endothelial progenitor cells (EPCs) or HemSC, which differentiate into GLUT-1–positive endothelial cells under the influence of mediators of EPCs and vasculogenesis (e.g., VEGF-A, VEGFR-2, and hypoxia-inducible factor [HIF-1α]) [11,13,16,28-30]. Increased VEGF and HIF-1α levels are observed in IH patients [13,27]. The second hypothesis is the placental theory, which suggests that IH is derived from embolized placental cells [13,28,29]. GLUT-1 expression in IHs, placental syncytiotrophoblast microvilli, and placental basement membranes and the clinical characteristics of rapid proliferation followed by gradual involution in IHs, which are similar to placentae, support this placental cell embolization theory [13,28,29]. The third hypothesis is the tissue hypoxia-induced vascular proliferation theory, which suggests that the induction and proliferation of EPCs are stimulated by hypoxic conditions (preterm, low birth weight, increased maternal age, placental anomalies) mediated by HIF-1α [13,28-30]. Tissue hypoxia induces neovascularization and angiogenesis by stimulating the expression of angiogenic factors such as VEGF and basic fibroblast growth factor (bFGF), on EPCs [5,7,11,21,27]. VEGF may be the most potent angiogenic factor involved in the pathogenesis of IH, stimulating endothelial cell proliferation and mobilization through matrix metalloproteinases [27]. The fourth hypothesis is the renin-angiotensin system theory, which suggests that the proliferation of IH-derived blast cells is induced by angiotensin (AT) II stimulated indirectly by high renin levels. Renin converts angiotensinogen to AT I, which leads to high levels of AT II [7,28]. High renin levels in infants and high-risk groups of IH patients and the expression of angiotensin-converting enzyme and AT II receptor 2 on the endothelium of proliferating IHs support this theory [28]. The mechanism of β-adrenergic blockers inducing the accelerated involution of IHs may be associated with the blocking of β1-adrenergic receptors in the kidney, leading to the inhibition of renin release [7,28].
Clinical characteristics of IH
IHs are not present at birth, usually develop during the first 1–2 weeks of age, rapidly proliferate during the first 1–3 months of age, finish proliferating at 5 months of age, and then spontaneously and slowly involute into the adipose and fibrous tissue until around 4 years of age but sometimes up to 10 years of age [9,11-15,26,31,32]. Segmental IHs and large focal IHs, especially the deep type or those located in the parotid gland, may show an extended growth period late during the second and third years of age [11,13].
IHs are most frequently located in the head and neck region (60%), followed by the trunk (25%) and the extremities (15%) [26]. IHs usually develop in the skin involving the epidermis, dermis, and subcutaneous fat but rarely in the internal organs such as the liver, gastrointestinal tract, respiratory tract, brain, or other organs. Large visceral IHs, especially hepatic IHs, may be complicated by hypothyroidism due to inactivation of thyroid hormones by type 3 iodothyronine deiodinase in IH tissues [33]. Visceral hemangiomas are usually associated with large segmental or multifocal cutaneous IHs [16].
Depending on the depth of the lesion from the skin’s surface, IHs are classified as superficial, deep, or mixed (superficial+deep) types or reticular/abortive/minimal growth types [1,11,16,26]. Superficial IHs are usually located in the epidermis and dermis with little or no involvement of the subcutaneous fat, bright red in color, and formally termed “strawberry hemangiomas.” [11] Deep IHs are located deep below the skin’s surface, more diffuse, and less well-defined than the superficial type, covered with skin, may be a normal skin color or have a bluish hue, and are formally termed “cavernous hemangiomas.” [11] The terms “strawberry hemangiomas” or “cavernous hemangiomas” are not used to describe IHs anymore [11]. Mixed or combined type IHs are hemangiomas containing both superficial and deep components. Deep type IHs or mixed type IHs with deeper soft tissue components often present at later age such as 1-2 months of age or later [11]. There is a subset of IHs with minimal or arrested growth (IH-MAGs) that typically present as a patch of reticulated telangiectasia and can be misdiagnosed as a port-wine stain or other vascular birthmark [11,34]. IH-MAGs can be complicated by ulceration or even structural anomalies if showing segmental patterns [11].
Depending on the anatomic appearance, IHs are described as having localized or focal, segmental, indeterminate, or multifocal patterns [1,11,16,26]. Localized or focal IHs are the most common pattern, well-circumscribed focal lesions appearing to rise from a point, and segmental IHs are plaque-like extensive IHs measuring often larger than 5 cm in diameter [16]. Indeterminate IHs are not clearly localized or segmental and often called partial segmental [16]. Multifocal IHs are multiple discrete IHs located at multiple distant sites [16].
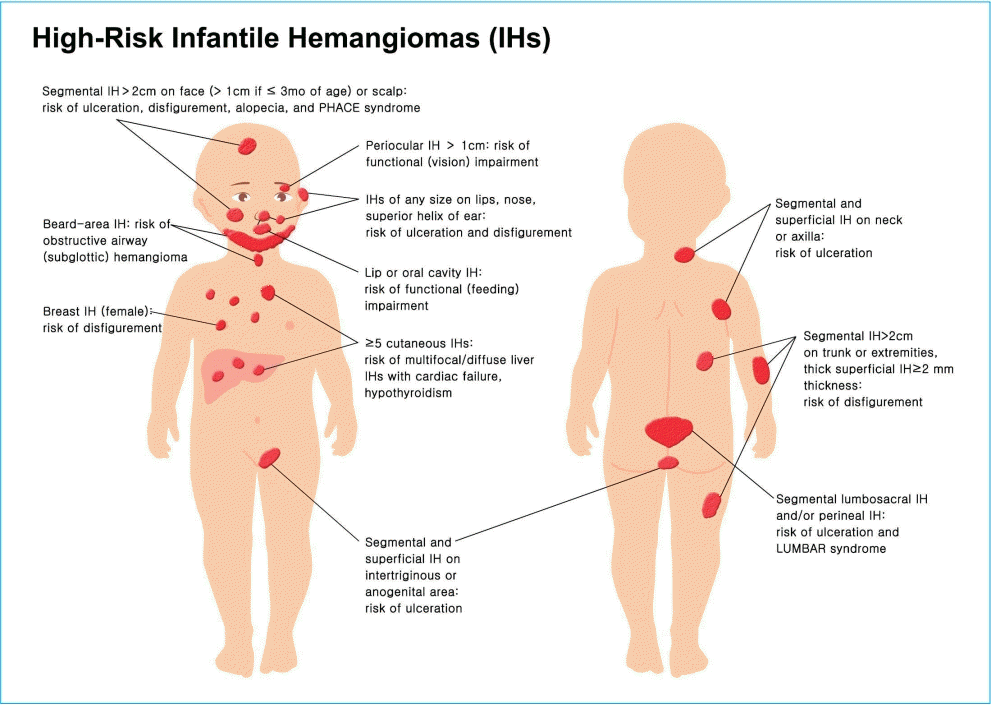
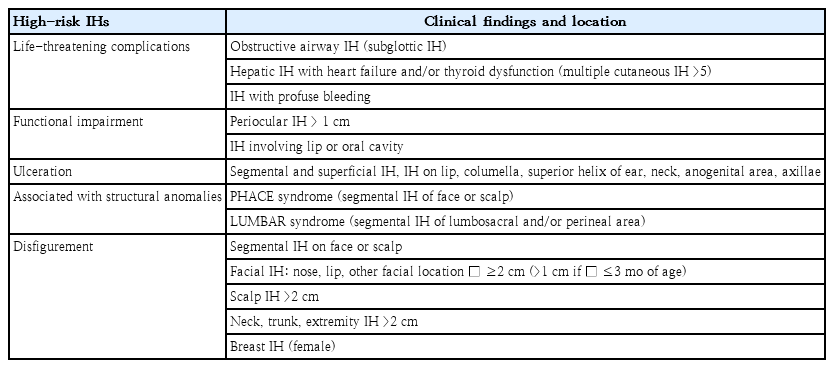
High-risk group IH
Primary care clinicians should identify the high-risk IH group before 3 months of age, ideally at 1 month of age, and transfer them to hemangioma specialists for further evaluation and early treatment. Potentially problematic or high-risk IH groups with consideration for early treatment are IHs those with: (1) life-threatening complications, (2) functional impairment, (3) ulceration, (4) associated structural anomalies (upper body [PHACE syndrome], lower body [LUMBAR syndrome]), or (5) possible permanent disfigurement (Table 3) [9,11-13].
IHs with life-threatening complications include obstructive airway hemangiomas such as subglottic hemangiomas (Fig. 1) [35-38], hepatic IHs associated with high-output congestive heart failure and hypothyroidism [39,40], and profuse bleeding from ulcerated IHs [11]. Subglottic hemangiomas are diagnosed at mean age of 4 months, and patients present with progressive biphasic stridor, a barking cough, and dyspnea, and they may be misdiagnosed with congenital laryngeal stridor or infectious croup [11,13,16]. Approximately 50% of subglottic hemangiomas have a cutaneous hemangioma [11]. Segmental IHs of the lower face (“beard distribution”) or anterior neck and oral and/or pharyngeal mucosal IHs are risk factors for obstructive airway hemangiomas [11-13,16]. Hepatic hemangiomas present as focal, multifocal, or diffuse types [8,11]. Hepatic IHs usually present as a multifocal or diffuse hepatic distribution, whereas congenital hepatic hemangiomas present as a focal hepatic lesion [8]. Multiple IHs are usually asymptomatic, but some are associated with high-flow macrovascular shunting causing high-output cardiac failure [8,11,13]. Diffuse hepatic IHs are rare but present as severe hepatomegaly leading to abdominal compartment syndrome causing abnormal ventilation and renal failure due to renal vein compression [11-13,39-41]. In cases of multiple or diffuse hepatic IHs, hypothyroidism can occur due to inactivation of the thyroid hormones by type 3 iodothyronine deiodinase in the IH tissue; therefore, thyroid function testing is required [8,11,13,16]. Hepatic IHs are usually associated with ≥5 multifocal cutaneous IHs [8,13,16].
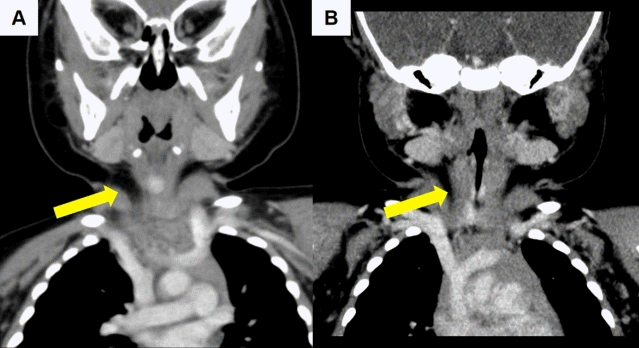
A 6-month-old boy diagnosed with subglottic laryngeal and superficial type cutaneous infantile hemangioma at diagnosis and after treatment with oral propranolol. He presented with chronic inspiratory stridor that had developed at 1 month of age and progressively aggravated until 6 months of age. Inspiratory stridor disappeared after 3 days of oral propranolol treatment at a dosage of 3 mg/kg/day #2. Computed tomography scan of subglottic laryngeal hemangioma (A) at diagnosis: 1.3 cmx0.7 cmx0.85 cm; and (B) after 1 month of treatment with oral propranolol: 0.8 cmx0.7 cmx0.8 cm (indicated with yellow arrows).
IHs with possible functional impairments are periocular, nasal, neck, lip, or oral cavity IHs (Fig. 2) [11,13,16]. Functional impairments such as visual disturbances, including mechanical ptosis, strabismus, anisometropia, or astigmatism, leading to the development of amblyopia, may occur in periocular IHs, especially in cases of upper eyelid IHs larger than 1 cm [11-13,42-44]. Functional impairments such as feeding problems may occur in cases of lip, oral cavity, or airway IH, leading to failure to thrive [11,13,16,45].
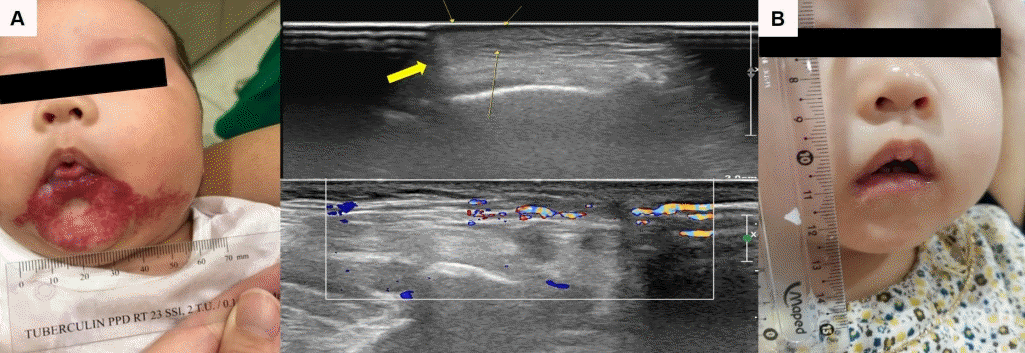
Infantile hemangioma (IH) causing possible functional impairments. (A) A 1-month-old girl at the diagnosis of lip, chin and oral cavity (tongue, palate, gingiva) IH who presented with feeding difficulty (gross and ultrasonography with Doppler: indicated with yellow arrows). (B) Improved state of IH after 12 months of treatment with oral propranolol at dosage of 2 mg/kg/day #3. Written informed consent was obtained from patient's parents for publication.
Skin or mucosal ulceration is the most common complication of IHs, occurring in 5%–21% of referred IHs and causing pain, bleeding, secondary infection, scarring, and disfigurement [46,47]. Ulceration is the most common complication of IHs, occurring in 10% of IHs and 15%–25% of referred IHs [11,13,16]. Ulceration usually develops in infants younger than 4 months of age during the period of rapid proliferation, frequently in superficial or mixed types or segmental IHs located on the scalp, neck, ear helix, perioral, perineal, perianal, and intertriginous areas and can cause pain, bleeding, infection, and scarring [11,13,16].
IHs with structural anomalies include PHACE syndrome and LUMBAR syndrome [11,16]. PHACE syndrome or PHACES is a congenital anomaly with IHs characterized by posterior fossa brain malformations such as Dandy-Walker malformation or cerebellar hypoplasia (52%), hemangioma of large (diameter >5 cm) segmental IHs involving the face, scalp, and/or neck (100%), arterial anomalies such as cerebrovascular aneurysms or stroke (90%), cardiac anomalies or coarctation of the aorta (67%), eye anomalies, and midline defects such as sternal cleft and/or supraumbilical raphe [11-13,16,48,49]. LUMBAR syndrome is another congenital anomaly with large segmental IHs involving the lower half of the body, such as the perineal, gluteal, or lumbosacral skin, often extending onto a unilateral lower extremity, undergrowth or overgrowth of the affected extremity, urogenital anomalies and ulceration, myelopathy, bony deformities, anorectal malformations, arterial anomalies, and renal anomalies [11-13,16,50].
IHs with permanent disfigurement usually occur in large-sized and facial IHs via rapid proliferation of IHs and scarring or distortion of landmarks such as the nose or lip [11-13]. Segmental IHs on the face and scalp; facial IHs in the nose or lips; any facial IHs ≥2 cm or > 1 cm in an infant ≤3 months of age; those >2 cm on the scalp, neck, trunk, or extremity; superficial IH ≥2 mm in thickness; or on the breast in female infants requires treatment to prevent permanent disfigurement (Fig. 3) [11-13].
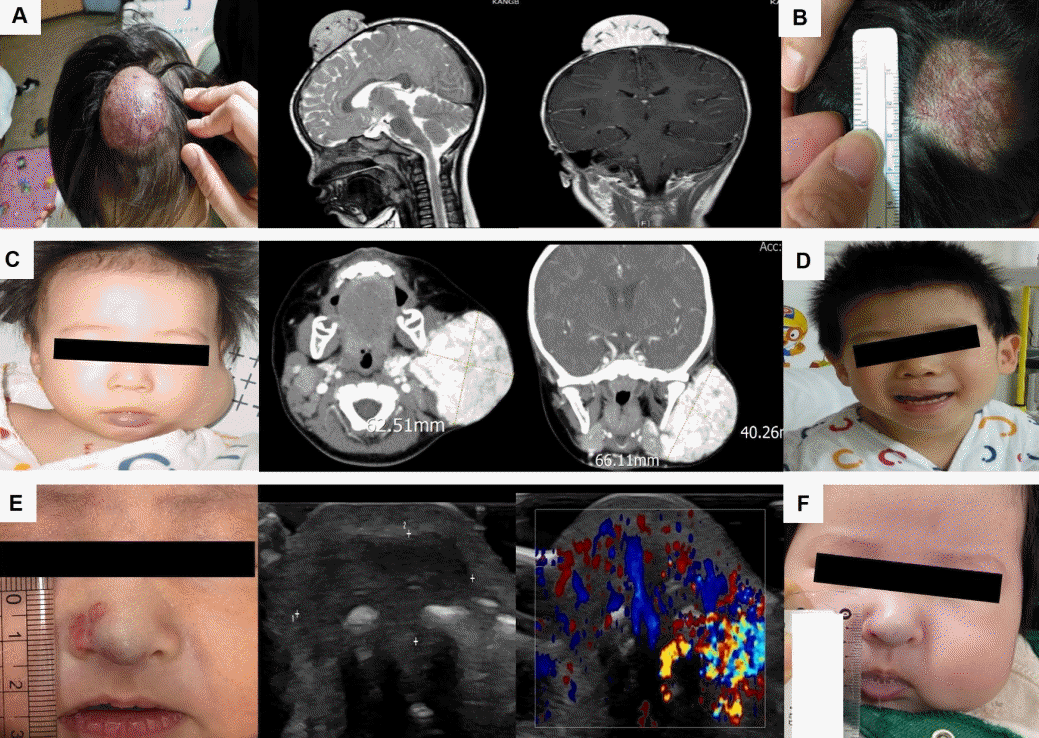
Infantile hemangiomas (IHs) causing possible permanent disfigurement. (A) 17-month-old girl at the diagnosis of a large scalp IH with long diameter of 4 cm and thickness of 2 cm (gross and magnetic resonance imaging [MRI]). (B) Improved state of scalp IH after 12 months of treatment with oral propranolol at a dosage of 2 mg/kg/day #3. (C) A 9-month-old boy at the diagnosis of a large left cheek IH with long diameter of 6.6 cm and thickness of 4.0 cm (gross and MRI). (D) Improved state of cheek IH after 12 months of treatment with oral propranolol at a dosage of 2 mg/kg/ day #3. (E) A 52-day-old girl at the diagnosis of nose IH (gross and ultrasonography with Doppler). (F) Improved state of the nose IH after 1 month of treatment with oral propranolol at a dosage of 3 mg/kg/day #2. Written informed consents were obtained from patients' parents for publication.
Diagnosis of IH
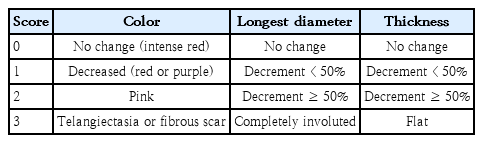
Most IHs can be diagnosed clinically through history taking and physical examinations. At the first clinic visit, the documented history should include birth history (gestation age, delivery type, birth weight, parity, single or twin pregnancy, maternal age), date (age) of onset or detection of hemangioma, period of proliferative growth, age at when the hemangioma stopped growing and started to regress, and the occurrence of complications such as ulceration or bleeding or infection (oozing) [11,16,19-22]. A physical examination should be performed initially and at every outpatient clinic visit at 1- to 3-month intervals and include vital signs, body weight and height, systemic physical examinations, and the details of the hemangiomas such as location, number, color, long diameter, thickness, consistency, and signs of involution to the adipose and fibrous tissue [12,19-22,51]. Hemangiomas should be photographed serially at the first visit, at every follow-up visit, and at 3 and 6 months after the discontinuation of treatment for comparison [12-14,19-22]. Response to treatment can be evaluated clinically using the visual analogue scale (VAS) for superficial IHs for color, longest diameter, and thickness, with each score ranging from 0 (indicating no change) to 3 (indicating complete resolution) (Table 4) [16,21,22,52]. Therapeutic outcomes are graded as excellent response (VAS 7–9), good (VAS 4–6), poor response (VAS 1–3), and no response (VAS 0) [21,22]. The VAS scores are evaluated during treatment and at the last follow-up [21,22].
US with Doppler is the imaging modality of choice for IH assessment and is recommended when the diagnosis of IH is uncertain, when IHs show explosive or prolonged growth without involution, to differentiate IHs from malignant vascular tumors or vascular malformations, when there are 5 or more cutaneous IHs, or when the association with anatomic abnormalities such as PHACE syndrome or LUMBAR syndrome is suspected [5,11-13,16]. US with Doppler, a noninvasive modality without ionizing radiation exposure, can be performed on neonates and infants without sedation to efficiently assess IH size, extent, characteristics (cystic or solid), and vascularity [11,16].
On US with Doppler, IHs appear as well-defined solid masses with high-flow vascularity but without arteriovenous shunting (Table 5) [3,5]. US with Doppler is also the imaging modality of choice for screening hepatic IHs, especially in cases of 5 or more cutaneous IHs, and for monitoring involution response to treatment [5,8,11-13,16,41,53].
MRI is recommended for infants with associated anatomic abnormalities such as PHACE syndrome or LUMBAR syndrome. Infants with a large (>5 cm in diameter) segmentally patterned facial or scalp IHs are at risk for PHACE syndrome, and MRI and/or MRA of the head and neck (including the aortic arch and brachiocephalic origin) and echocardiography are recommended for further evaluation [5,16,48]. Infants with large segmental IHs on the lower half of the body are at risk for LUMBAR syndrome, for which spine MRI is recommended, especially in the presence of sacral dimples, skin appendages, hair tufts, or lipomas. US and Doppler of the spine, pelvis, and abdomen can be performed to initially screen for LUMBAR syndrome in young infants with a corrected age of less than 6 months, but ultimately more sensitive MRI will be required to ensure the accurate diagnosis of spinal abnormalities [5,50]. On MRI, IHs appear as well-delineated homogeneous solid masses with iso- to intermediate signals on T1-weighted images and intermediate to bright signal intensity on T2-weighted and proton density-weighted images (Table 5) [5,11-13,16].
Biopsy and pathological diagnosis are rarely required when the hemangioma lesion is differentiated from a borderline or malignant vascular tumor [8,11].
Differential diagnosis of IH
IHs must be differentiated from other vascular tumors, including benign vascular tumors such as CHs, borderline vascular tumors such as Kaposiform hemangioendothelioma (Fig. 4), and malignant tumors such as angiosarcoma; their differences in pathology, clinical findings, imaging, and treatment are summarized in Table 5 [3,5,25,26]. IHs must also be differentiated from vascular malformations; their differences in clinical characteristics, imaging, pathology, and treatment are summarized in Table 6 [3,8,25,26].
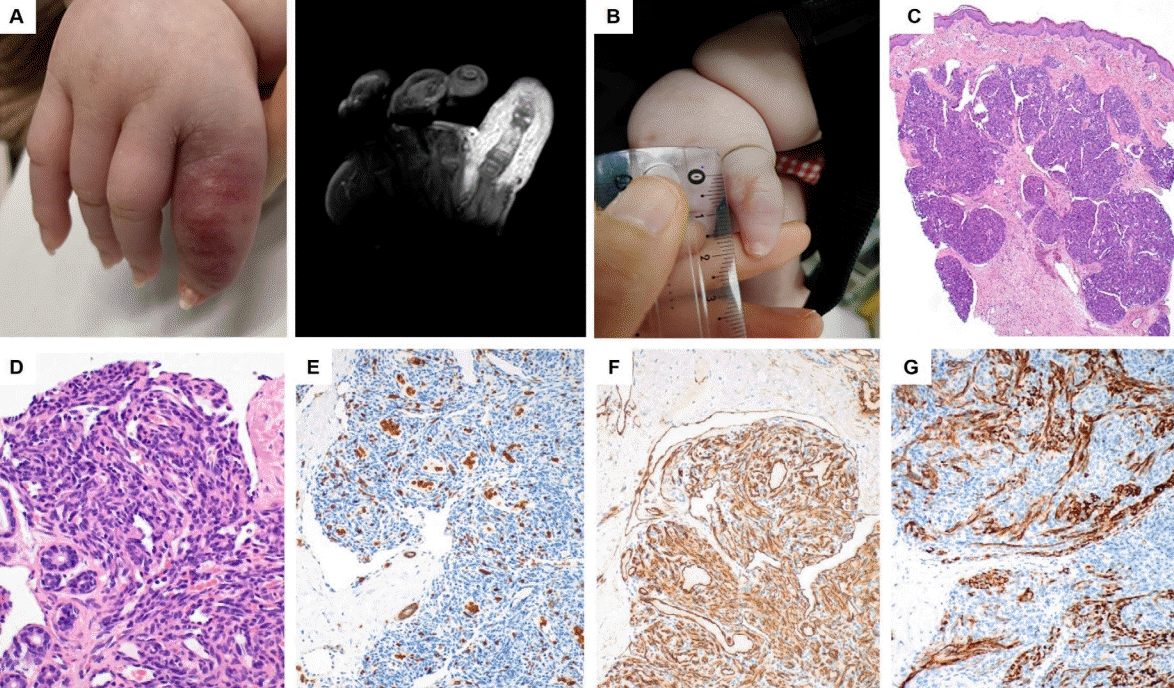
Kaposiform hemangioendothelioma (KHE) on the left little finger. (A) A 1-month-old girl presented with a rapidly enlarging hemangioma of the left little finger that developed at birth (gross and magnetic resonance imaging). (B) Improved state of KHE after 1 month of treatment with oral propranolol and oral corticosteroid (prednisone). (C) Pathology show hypervascular and lobulated nodules infiltrating the dermis (hematoxylin-eosin, original magnification, ×40). (D) High power view showing closely packed spindleshaped tumor cells with slit-like vascular lumens (hematoxylin-eosin, original magnification, ×400). (E) Immunohistochemical stains show negativity for glucose transporter-1 protein (original magnification, ×200). (F) CD34 stain is positive for the tumor cells (original magnification, ×200). (G) D2-40 stain is positive for the neoplastic spindle cells of the peripheral portion of the lobules and small vascular channels (original magnification, ×200). Written informed consent was obtained from patient's parents for publication.
1. Congenital hemangioma
CHs are rare benign vascular tumors that differ from IHs, are characteristically present at birth as fully developed hemangioma, do not show a proliferative phase or growth after birth, and are pathologically GLUT-1–negative (Table 5) [3,5,25,26]. CHs present as less well-defined bluish-red plaques with telangiectasia on the surface or as a raised greyish tumor surrounded by a pale halo with multiple telangiectasia [5,25,26]. RICH undergo a rapid involution phase and completely regress by 12–18 months of age, leaving fibrofatty residual tissue behind [5,25,26]. NICH do not show an involution phase; rather, they remain stable or grow proportionately with the child [5,25,26]. Some CHs will decrease in size to a certain point and then remain stable and are known as PICH [5,25,26]. Other CHs, especially RICH, are extensive at birth and can be complicated by high-output cardiac failure, thrombocytopenia, and consumption coagulopathy, requiring early treatment. RICHs are treated the same as IHs, while NICH and PICH remain untreated or are surgically resected [3,5,26].
2. Vascular malformations
Vascular malformations are congenital vascular defects of vascular morphogenesis caused by dysfunction during embryogenesis and vasculogenesis. Vascular malformations are present at birth, may not be detectable clinically, and do not show a proliferative or involutive phase after birth; rather, they grow proportionately with the child or expand hemodynamically due to infection, trauma, or hormonal changes (puberty, pregnancy) and are pathologically GLUT-1–negative. Vascular malformations can be diagnosed by physical examination, US with Doppler, MRI, and MRA (Table 6) [3,5,8,10,26].
Capillary malformations (CMs) develop most commonly on the head and neck, are usually unilateral, and appear as well-circumscribed pink to purple macular lesions of variable size. CMs may occur as a component of Sturge-Weber syndrome, Klippel-Trenaunay syndrome, and other syndromes such as CLOVES and Beckwith-Wiedemann (Table 1) [1,3,10]. CMs are treated with PDL beginning in infancy [17,18].
VMs appear as nodular type masses of venules that diffuse to large superficial (resembling varicose vein) or deep VMs (Fig. 5) [2-5,7,8,10]. Chronic slow-flow VMs are at high risk of localized intravascular coagulation with consumption coagulopathy, which may be aggravated by infection, trauma, or hormonal changes (puberty, pregnancy) [3,8,10]. Large VMs may be associated with pulmonary emboli [8]. Painful or symptomatic VMs may be treated with sclerotherapy with the administration of sclerosing agents (OK432 [Picibanil], ethanol, bleomycin, sodium tetradecyl sulfate), sirolimus (mammalian target of rapamycin inhibitor), surgical excision, laser ablation, or embolization [3,5,8,27,54-56]. The use of low-molecular-weight heparin is recommended in highrisk VM patients before and after the procedure to reduce both hemorrhagic and thrombotic complications [8]. Compression stockings (20–30 mmHg), hydrotherapy, and lymphatic massage can be used as adjunctive therapy [3,8]. Oral contraceptives are contraindicated in patients with VMs due to the thrombosis risk [8].
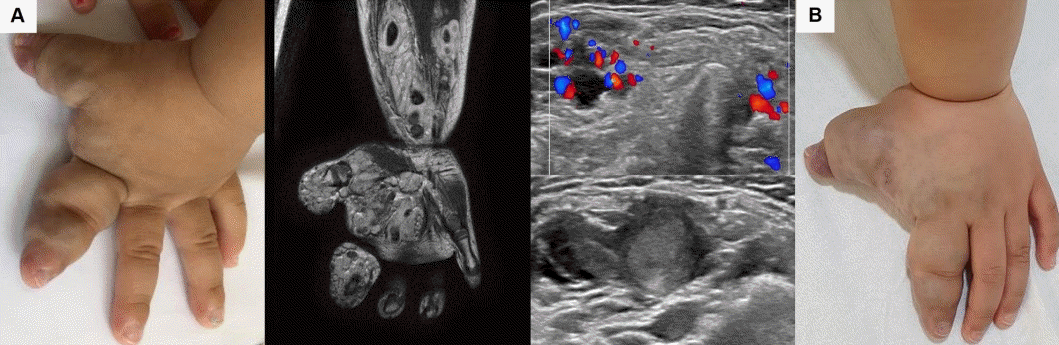
A 3-year-old boy diagnosed with venous malformation (VM) of the left hand complicated by localized intravascular coagulopathy (LIC). The VM was present at birth and grew with the child, accompanied by increasing pain. (A) VM with LIC at the time of diagnosis (gross, magnetic resonance, and ultrasonography with Doppler), (B) VM with LIC after 12 months of treatment with oral propranolol at a dosage of 2 mg/kg/day #2. After treatment, the VM did not decrease in size, but the pain subsided. The patient is able to do exercises using both hands freely as well as play piano. Written informed consent was obtained from patient's parents for publication.
Lymphatic malformations (LMs) are malformation of the lymphatic channels that consist of dilated lymphatic channels or cysts lined by lymphatic endothelial cells, and occur most frequently in the head, neck, and axilla [2-5,7,8]. LMs are classified as macrocystic, microcystic, or mixed depending on cyst size [1]. Localized macrocystic LMs are effectively treated with sclerotherapy with sclerosing agents (doxycycline, OK432, ethanol, bleomycin, sodium tetradecyl sulfate), those involving the skin and mucosa may be treated with PDL, and those that are complicated or extensive can be treated with sirolimus alone or in combination with sclerotherapy [3,5,8,27,55-57].
AVMs are direct connections of arteries to veins that bypass the capillary bed [2-5,7,8]. AVMs of the skin are very rare, present as reddish-pink patches progressing to a deep red color with skin thickening, and can be diagnosed by arterial palpation. As some AVMs are progressive, leading to significant morbidity and even mortality, an early diagnosis and multidisciplinary team approach are necessary. AVMs and arteriovenous fistula are treated with embolization and/or surgery [3,5,17,18,27].
Treatment of IH
The majority of IHs involute spontaneously and do not require treatment, especially when small, superficial, located in areas covered by hair or clothing, or unlikely to cause disfigurement or other complications [5,11]. However, primary care clinicians should actively observe neonates or infants with hemangioma at frequent intervals during the first few weeks and months of life and educate parents about the natural course, complications, and psychosocial impact of IHs [11-13]. As recent studies show that most IHs complete the proliferative phase by 5 months of age, the 2019 AAP CPG for IH management recommends that primary care clinicians refer infants with high-risk IHs to IH specialists by 1 month of age [11,14,15]. The IH management guideline from Europe, the US, Australia, and Japan all recommend oral propranolol as the first-line treatment for high-risk IHs [11-13]. The 2019 AAP CPG for IH management recommends topical timolol maleate application for small superficial IHs. In the Agency for Healthcare Research and Quality review of 2 large prospective multicenter randomized controlled trials (RCTs) and 4 cohort studies, the efficacy or estimated expected clearance of IHs was 96% when treated with propranolol, 62% when treated with topical timolol, 58% when treated with intralesional triamcinolone, and 43% when treated with oral steroids compared to 6% in the control group [9,11].
1. Oral propranolol
Propranolol, a nonselective beta-adrenergic receptor antagonist, has been used for several decades in the treatment of hypertension, ischemic heart disease, arrhythmias, heart failure, thyrotoxicosis, migraine, and glaucoma at a pediatric dosage of 1–5 mg/kg/day and maximum dosage up to 8–16 mg/kg/day [11,13]. After the first report on the successful treatment of IHs with oral propranolol [58] followed by a large prospective multicenter RCT of 456 young infants with IHs in Europe and the US by Léauté-Labrèze et al, oral propranolol hydrochloride solution was approved by the U.S. Food and Drug Administration (FDA) and European Medicines Evaluation Agency in 2014 and Korean FDA in 2016 for the systemic treatment of proliferating IHs [51,59]. Oral propranolol therapy is now the gold standard therapy and first-line treatment for proliferating high-risk IHs [9,11-13,16-18,51,58,59]. Propranolol hydrochloride is available in solution and tablet form, and its recommended dosage for IHs is 2–3 mg/kg/day divided into 2 daily doses, although the best outcomes occur at 3 mg/kg/day divided into 2 daily doses when maintained for at least 6 months and often continued until 12 months of age or occasionally longer depending on IH size without increased side effects (Table 7) [9,11-13,16,59,60]. For most IHs, oral propranolol treatment ends around 12 months of age, but in very complex cases, treatment may last longer [12]. Some clinicians wean propranolol over 2 weeks to months [11,13,16], but propranolol can be discontinued safely without weaning [12].
The mechanism of action of propranolol on IHs is unclear; however, the hypothesized mechanisms are the induction of vasoconstriction, angiogenesis inhibition by decreased VEGF and bFGF levels, induction of endothelial cell apoptosis, inhibition of nitric oxide production, and suppression of the renin-angiotensin system [27,28,61-67].
The rare but serious side effects of propranolol are bradycardia (0.1%), hypotension (0.1%), hypoglycemia (0.6%), bronchospasm, and bronchial hyperreactivity (0.9%–12.9%) [9,11-13,16,27,51,68-74]. The common but nonserious side effects of propranolol are sleep disturbances (2%–18.5%), somnolence, irritability, diarrhea, constipation, and cold extremities [27,51,69,72-76].
Contraindications to propranolol therapy and indications for its inpatient initiation are summarized in Table 7 [9,11-13,16]. In inpatient setting, the dosage is initiated at 1 mg/kg/day divided into 2 doses with physical examination (pulmonary auscultation, liver palpation, neurodevelopment), heart rate (HR), and blood pressure (BP) monitoring at 1 and 2 hours after each dose. If the 1 mg/kg/day dose is tolerated, the dose is increased daily to 2 mg/kg/day divided into 2 doses the next day and finally to 3 mg/kg/day divided into 2 doses with the continuation of HR and BP monitoring at 1 and 2 hour after each dose [9,11-13,16,19,20,51]. In the outpatient clinic setting, the dosage is initiated similarly as in the inpatient setting at 1 mg/kg/day divided into 2 doses on day 0, increase to 2 mg/kg/day divided into 2 doses on day 7, and finally to 3 mg/kg/day divided into 2 doses on day 14 with the similar physical examination, HR, and BP monitoring for 2 hours after the initiation dose and at day 7 and day 14 visits for the dosage increases [11,13,16,51,59]. In both inpatient and outpatient settings, electrocardiography monitoring is recommended for patients with a history of arrhythmia and/or bradycardia, and blood glucose monitoring is recommended for infants who were preterm or small for gestational age, had failure to thrive, or had a history of hypoglycemia [11,13,51]. In all situations, propranolol doses must be given 9–12 hours apart during or after feeding to minimize hypoglycemia [11,13,16,59]. Propranolol therapy should be temporarily stopped if the child is in a state of prolonged fasting due to illness or vomiting [11,13,16]. As propranolol is metabolized mainly by the liver, a serum aspartate aminotransferase and alanine aminotransferase evaluation is required before its initiation [51]. IHs usually respond with growth arrest and the start of involution within 2–4 weeks of oral propranolol treatment initiation [9,11-13,19,20,22,59].
Propranolol is superior to oral captopril at treating problematic IHs and to ibuprofen and paracetamol at treating ulcerated IHs [11,77]. No significant difference in efficacy was reported between the lipophilic nonselective beta-blocker propranolol and the hydrophilic selective beta-1 blocker atenolol [9,11,78].
2. Topical timolol maleate
Topical timolol maleate, a nonselective β-adrenergic receptor antagonist, has been used for several decades in the treatment of pediatric glaucoma, and since 2010 in the treatment of IHs [9,16,52,79-85]. The 2019 AAP CPG for IH management states that timolol maleate 0.5% gel-forming ophthalmic solution may be topically applied to small, thin, and superficial IHs at a dose of 1–2 drops applied twice daily for 6–9 months until 12 months of age or occasionally longer depending on IH size [11-13]. A reported 92.3% of IH patients showed improved lesion color, size, extent, and volume when treated with topical timolol for 6–9 months [79]. Timolol can be detected in the blood or urine of some IH infants [86,87]. Adverse events including local irritation (mostly) and bronchospasm were observed in 3.4% of patients, and no patients discontinued timolol as a result [84,85].
3. Corticosteroids
Systemic corticosteroid therapy may be considered in IH patients in whom propranolol is contraindicated or ineffective [9,11-13,16]. However, it is not recommended in cases of adverse events [9,11-13,16]. Oral prednisolone or prednisone at a dosage of 2–5 mg/kg/day divided into 3 daily doses for 4–12 weeks followed by gradual tapering and completion of therapy by 9–12 months of age have been used [80]. Treatment consisting of low-dose corticosteroids and propranolol is recommended for PHACES syndrome, which features an increased risk of stroke [11,13]. Some small localized IHs on anatomic landmarks such as the lip or nose have been treated with the intralesional injection of triamcinolone and/or betamethasone at 4- to 6-week intervals [88-90].
Corticosteroid therapy is consistently associated with adverse events such as Cushingoid appearance, infection, growth retardation, hypertension, and mood changes, which are very harmful to young infants [9,11,91]. In addition, intralesional triamcinolone may cause local complications such as fat and/or dermal atrophy and pigmentation [88-90]. Therefore, corticosteroid therapy is not recommended for young infants with IHs [9,11-13,16].
4. Other treatment
Sirolimus, an inhibitor of the mammalian target of rapamycin (mTOR), has potent antiangiogenic activity in Kaposiform hemangioendothelioma, complicated IHs, LMs, and VMs at a starting dosage of 0.8 mg/m2 with the goal of achieving a trough level of 7–9 ng/mL in the early morning [5,8]. More RCTs demonstrating its safety and efficacy for IHs are required [8].
Interferon therapy is not recommended due to serious side effects such as spastic diplegia (25%) [13]. Vincristine is not recommended either due to hematological side effects and the risk of peripheral neuropathy [13].
Laser treatment including PDL or surgical resection is not recommended as the first-line treatment for proliferating IHs [11-13,27]. PDL therapy or delayed surgery are recommended for the treatment of residual skin changes after IH involution [11-13]. PDL therapy targets hemoglobin within the lesion and avoids thermal injury to the surrounding normal tissue [16]. PDL penetrates only the superficial dermis and may be effective at diminishing redness, but it does not affect the deeper elements of IHs [11-13]. Pain, ulceration, atrophic scarring, and pigmentation may develop as side effects after laser therapy for IHs [11-13,27].
Prognosis of IH
Approximately 10%–20% of IHs may develop serious complications requiring treatment such as ulceration, infection, bleeding, functional impairment, permanent disfigurement, hypothyroidism, and airway obstruction [11-13,16]. An estimated 85%–90% of IHs involute spontaneously and do not need treatment, but 50%–70% of involuted IHs may leave behind residual skin changes such as telangiectasia, fibrofatty tissue, redundant skin, atrophy, scarring, hypopigmentation, or alopecia [11-13,16]. Among the referred untreated IH patients, 55%–69% show permanent disfigurement [11].
Rebound growth may occur in 10%–25% of treated IHs during tapering or after stopping propranolol, but they usually respond if treated again with propranolol [22,59,92]. Risk factors for IH relapse include discontinuation of therapy before 12 months of age, mixed or deep type, and female sex [92]. The best responses are observed when IHs are superficial, thin (<1 mm thickness), and in infants 6 months old or younger [9,11,59]. An estimated 7% of infants require subsequent treatment with oral propranolol [85].
Conclusions
As IHs are the most common benign tumor of infancy, developing in up to 10% of infants, and best diagnosed at 1 week to 1 month of age, primary care pediatricians are the first doctors to recognize and diagnose them. It is recommended that primary care pediatricians understand IHs and other VAs, monitor patients monthly during early infancy for IH growth and complications, and refer high-risk patients to IH specialists (pediatric hematology-oncologists in Korea) for treatment as early as 1 month of age. Oral propranolol therapy is currently the gold standard therapy for high-risk IHs.
See the commentary on "Infantile hemangioma: timely diagnosis and treatment" via https://doi.org/10.3345/cep.2021.00752.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.