Thrombosis and severe acute respiratory syndrome coronavirus 2 vaccines: vaccine-induced immune thrombotic thrombocytopenia
Article information
Abstract
The development of vaccines against severe acute respiratory syndrome coronavirus 2, which features high mortality and morbidity rates, has progressed at an unprecedented rate, and vaccines are currently in use worldwide. Thrombotic events after vaccination are accompanied by thrombocytopenia, and this issue was recently termed vaccine-induced immune thrombotic thrombocytopenia. This manuscript describes recently published guidelines and other related issues and demonstrates characteristic cases.
Key message
· Thrombosis and thrombocytopenia occurring within 4–28 days after severe acute respiratory syndrome coronavirus 2 vaccination require attention.
· The terms vaccine-induced immune thrombotic thrombocytopenia (VITT) and thrombosis with thrombocytopenia syndrome (TTS) are used.
· VITT is pathogenetically similar to heparin-induced thrombocytopenia.
· VITT/TTS could be associated with the development of platelet-activating anti-platelet factor 4 antibodies.
· For suspected VITT/TTS, early treatment decisions (intravenous immunoglobulin, non-heparin anticoagulant, and avoidance of platelet transfusions) are important.
Introduction
Coronavirus disease 2019 (COVID-19), an infectious disease caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can result in high mortality and morbidity [1]. The COVID-19 pandemic has created an ongoing global medical crisis.
Vaccines against SARS-CoV-2 comprise a crucial solution to overcoming this pandemic. By May 2021, the World Health Organization approved the emergency use of the COVID-19 vaccines developed by Pfizer/BioNTech, AstraZeneca (AZ), Janssen, Moderna, Sinopharm, and Sinovac [2]. Several other vaccines, including the Novavax and Sputnik V, are being prepared for approval. The U.S. Food and Drug Administration has approved 3 vaccines, 2 messenger RNA-based vaccines (Pfizer/BioNTech and Moderna) and an adenoviral-based vaccine (Janssen), for emergency use [3]. The European Medicines Agency approved the vaccines distributed by Pfizer/BioNTech, Moderna, Janssen, and AZ [4].
The development and approval speed of these vaccines against SARS-CoV-2 is unprecedented. Various issues have recently been raised. Inter alia, some cases of atypical thrombotic events after vaccination with the recombinant adenoviral vector encoding the spike protein antigen of SARS-CoV-2 has raised many concerns. The AZ vaccine consists of a replication-deficient chimpanzee adenoviral vector encoding the spike glycoprotein of SARS-CoV-2 [5], and the Janssen vaccine is a recombinant, replication-incompetent adenovirus type 26 vector encoding the SARS-CoV-2 spike glycoprotein [6].
This review discusses several cases and related details and provides various guidelines and recommendations.
Vaccine-induced immune thrombotic thrombocytopenia
Some cases of abnormal thrombotic events with thrombocytopenia were first identified in patients at the end of February 2021 after vaccination with the AZ vaccine. Thereafter, similar cases have been reported of thrombosis and thrombocytopenia after vaccination with AZ vaccine in April 2021 [7,8]. Symptoms common to these cases include the following: (1) thrombosis, especially in uncommon sites; (2) mild to severe thrombocytopenia; and (3) positive platelet factor 4 (PF4)-heparin enzyme-linked immunosorbent assay (ELISA) and platelet activation assays, and these symptoms usually appear between about and 4–28 days after COVID-19 vaccination based on adenoviral vector. The syndrome showing these symptoms has recently been referred to as vaccine-induced immune thrombotic thrombocytopenia (VITT), thrombosis with thrombocytopenia syndrome (TTS), or vaccine-induced prothrombotic immune thrombocytopenia (VIPIT) [7-13]. Reports to date stated that 95%–100% of cases occur after the first dose of the vaccine [14].
In a report of 5 cases in Norway [8], thrombosis accompanied by thrombocytopenia occurred in patients aged 32–54 years 5–10 days after vaccination with the AZ vaccine. The affected sites were unusual and included the cerebral venous sinus, portal vein, and/or splanchnic vein; 3 of the 5 patients died. None had previously been administered heparin, and antibodies to the PF4-polyanion complexes were identified in all of them.
A United Kingdom report [7] stated that 11 patients for whom clinical data were available had one or more thrombotic complications post vaccination with AZ vaccine. The median patient age was 36 years (range, 22–49 years); 9 of them were women. All showed varying degrees of thrombocytopenia (median trough count, 20,000/mm3; range, 9000–107,000/mm3). No patient received heparin prior to the event. In all samples of 4 of these 11 patients and 24 patients with similar thrombotic events, the PF4-heparin and PF4 immunoassay results were positive and showed PF4-dependent platelet activation.
Similar cases that occurred after administration of the Janssen vaccine were also reported in the United States [15]. A previously healthy 48-year-old White woman visited the Emergency Department with a 3-day history of malaise and abdominal pain. She had been vaccinated with the Janssen vaccine 14 days before symptom onset. The laboratory results showed marked thrombocytopenia (platelet count, 13,000/mm3), a low fibrinogen level, a prolonged activated partial thromboplastin time, and severe elevation in the D-dimer level. Computed tomography of the abdomen and pelvis revealed extensive splanchnic-vein thrombosis. Up to this point, her condition has not resolved.
No thrombotic signs were detected in clinical trials of the AZ vaccine [5], which has been administered to 34 million people worldwide to date. The phase 3 trial of the Janssen vaccine included 19,630 vaccinated participants and 19,691 placebo participants [6]. Venous thrombotic events were observed in 11 participants in the vaccine group and 3 participants in the placebo group. The types of thrombosis were deep vein thrombosis (DVT), pulmonary embolism, and transverse sinus thrombosis. In this report, the authors stated that most participants who developed thrombosis had underlying medical conditions and that predisposing factors might have contributed to these events. Other adenoviral vaccines include the CanSino and Sputnik V, but there are no reports of VITT in patients who received this vaccine [9].
As of June 2021, the reported incidence was 242 of 22,600,000 in the United Kingdom, 28 of about 8.7 million in the United States, and 5 of 132,686 in Norway [8,15,16]. In Korea, it was reportedly 2 of 9.03 million people (Table 1) [17]. The incidence varies among countries [8,15-19]; in particular, the incidence in Korea is reportedly lower than that in other countries.
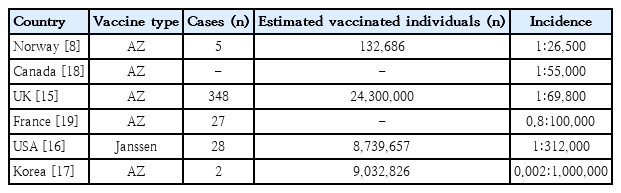
Published and reported incidence of vaccine-induced thrombotic thrombocytopenia in various countries
Not all mechanisms are known, but many of the clinical and laboratory features after vaccination with the AZ or Janssen vaccine resemble those observed in patients with immune heparin-induced thrombocytopenia (HIT), which is characterized by strong positivity of anti-PF4/heparin antibodies and leads to platelet activation (Table 2) [10,12,20]. PF4 is a chemokine stored in platelet alpha granules that are released when platelets are activated and binds polyanions with high affinity. Heparin may act as a hapten, i.e., when it binds to PF4, production of specific antibodies is spurred [21,22]. Although the mechanism is complicated, it is currently described as the development of antibodies against PF4/heparin complexes. HIT is a complication of heparin therapy. It occurs approximately 5–10 days after the first administration of heparin and carries a high risk of thrombosis. HIT occurs over 3 times more frequently in surgical and major trauma patients than in medical patients and is rarely observed in pediatric populations [21,23]. Platelet activation is also promoted by PF4 in HIT as in VITT. Autoimmune HIT with an atypical course may appear in a small number of cases without a history of heparin administration [21]. It may occur in cases of tissue damage, such as orthopedic surgery or inflammation due to infection [9,21,22]. Thrombocytopenia, thrombosis, and antiPF4 positivity may also mean that VITT may be an autoimmune type of HIT. It also explains that the interaction between the vaccine and platelets or between the vaccine and PF4 will affect its pathogenesis [7].
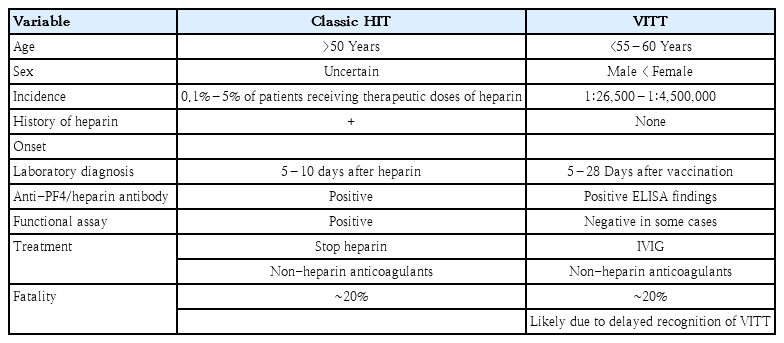
Clinical characteristics of classic heparin-induced thrombocytopenia (HIT) and vaccine-induced thrombotic thrombocytopenia (VITT)
Identifying circulating anti-PF4/heparin antibodies is a definitive diagnostic test [10,11]. It cannot be detected in commonly used methods such as latex immunoturbidity assay (LIA) [9,10,12]. LIA detects HIT antibodies based on competitive inhibition with the aggregation of latex particles coated with a monoclonal HIT-like monoclonal antibody. ELISA is the best diagnostic method. Therefore, if results have not yet been released but thrombosis has been accompanied by thrombocytopenia, appropriate treatment may be considered before the results are confirmed. Functional assays, such as the serotonin release assay, are also reportedly positive, but some patients test negative [24].
Based on these contents, various academic societies and organizations, including the International Society on Thrombosis and Haemostasis (ISTH), have presented the following guidelines (Fig. 1) [10-13]. If the following symptoms develop at 4–28 days after vaccination with the AZ or Janssen vaccine, a further diagnosis is required for the suspicion of VITT/TTS (e.g., severe, persistent headache; acute pain in the chest or abdomen; leg swelling or pain). Important examinations include a complete blood count analysis for checking platelet count, blood smear, D-dimers, and, depending on symptoms, appropriate imaging studies. If thrombocytopenia and acute thrombosis are confirmed, an immunoassay for the PF4 antibody should be performed. As mentioned above, HIT ELISA for anti-PF4/heparin antibody is the most reliable non-ELISA assay in VITT, and its sensitivity and specificity require further validation. A positive PF4 antibody immunoassay test, especially in cases of high optical density readings, suggests a high likelihood of VITT. If possible, treatment should be initiated immediately to confirm the appropriate assay for confirming PF4 antibody function.
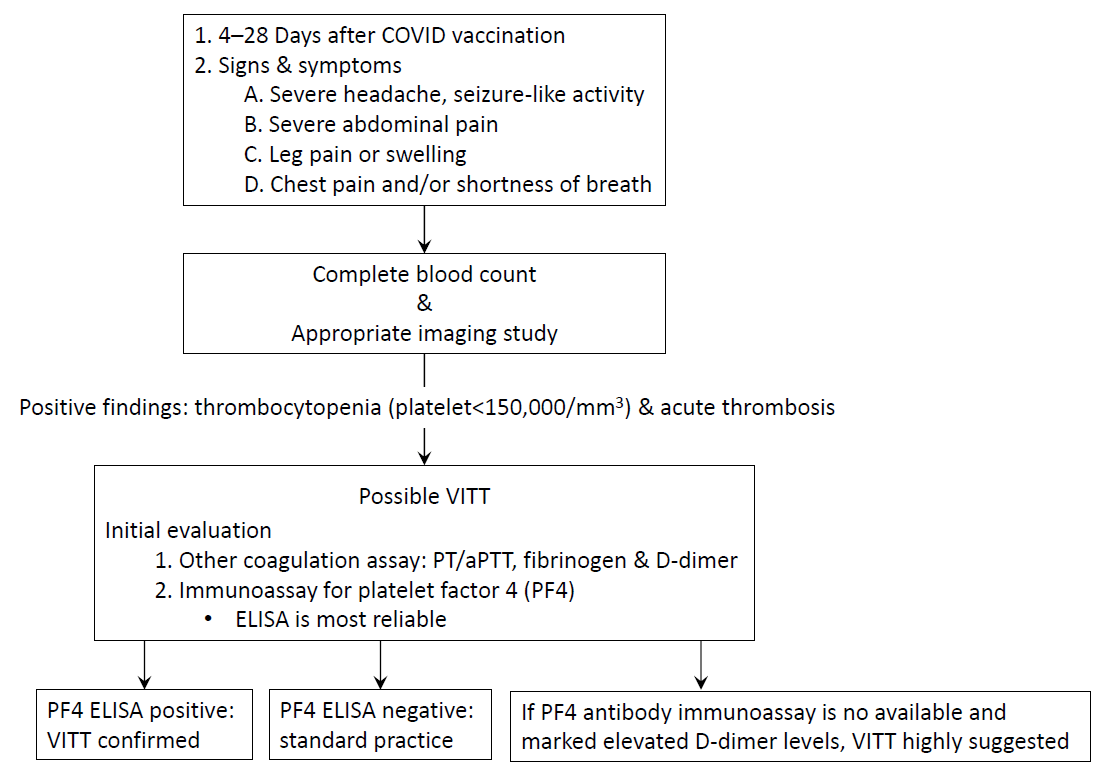
Diagnostic flow chart of VITT based on International Society on Thrombosis and Haemostasis Interim Guidance (updated 20 April 2021). In Korea, the PF4 ELISA is currently performed by sending a sample after reporting a suspected patient case to the Korea Disease Control and Prevention Agency. COVID, coronavirus disease; PT, prothrombin time; aPTT, activated partial thromboplastin time; PF4, platelet factor 4; ELISA, enzyme-linked immunosorbent assay; VITT, vaccine-induced immune thrombotic thrombocytopenia.
Treatment
If the diagnosis of VITT seems likely, treatment may need to be started before the full results are confirmed. Treatments suggested by the ISTH guidelines and other recommendations are as follows [10-13,25]: (1) intravenous immunoglobulin immediately (0.5–1 g/kg daily for 2 days), (2) consider steroids (e.g., prednisone 1–2 mg/kg) if the platelet count is less than 50,000/mm3; (3) avoid platelet transfusions (unless the patient requires urgent surgery), heparin, low-molecular-weight heparin, and vitamin K antagonists; and (4) administer a non-heparin anticoagulant such as fondaparinux or argatroban or a direct oral anticoagulant (e.g., apixaban, rivaroxaban) if the platelet count is >50,000/mm3 and there is no serious bleeding.
The treatments described above are constantly changing as new reports emerge. According to earlier reports that VITT was not clearly recognized, a patient’s course worsened after platelet transfusion and heparin administration. In earlier reports, the fatality rate was as high as 20% [7,8,25]; however, a recent report showed a successful course with a non-heparin anticoagulant, intravenous immunoglobulin, and steroid [26].
Other SARS-CoV-2 vaccines and thrombosis/thrombocytopenia
A case of thrombosis after the second dose of mRNA SARS-CoV-2 vaccine was reported [27]. A 66-year-old woman with no reported clinical problems received 2 doses of Pfizer/BioNTech vaccine. After 24 hours of the second vaccine dose, she experienced fever with chills, fatigue, and myalgia; 72 hours after the second vaccine dose, she was admitted for evaluation due to severe pain and walking difficulty. The presence of DVT involving the right peroneal vein that extended up to the popliteal vein was confirmed on color Doppler ultrasonography. The patient was started on apixaban as an anticoagulant and her symptoms resolved. In this case, PF4 antibody testing was not performed.
Secondary immune thrombocytopenia cases occurring after vaccination with an mRNA vaccine (e.g., Pfizer/BioNTech and Moderna vaccines) have also been reported [28,29]. According to one report, 20 cases of thrombocytopenia after vaccination were identified based on various data reviews, and the authors reported that the incidence of symptomatic thrombocytopenia after vaccination was much lower than the SARS-CoV-2 mortality and morbidity rates.
Coagulopathy in COVID-19, especially in children
High thrombotic complications have been noted in patients with COVID-19 infection, and this has recently been attributed to a complex and unique interaction between coronavirus and endothelial cells, local and systemic inflammatory responses, and coagulation systems [30]. According to several recent reports, in the most severe patients, coagulopathy and massive intravascular coagulation such as disseminated intravascular coagulation are frequently observed in patients. COVID-19–associated coagulopathy can be represented by elevated D-dimer and increased fibrin/fibrinogen degradation products, and these changes in hemostatic biomarkers indicate that the coagulopathy involves large-scale fibrin formation. However, the mechanism underlying coagulation remains unknown. It can be assumed that a dysregulated immune response is coordinated and controlled by inflammatory cytokines, lymphocyte apoptosis, hypoxia, and endothelial damage [31].
There are few reports of coagulopathy in children and adolescents infected with COVID-19. Recently, it has been reported that children and adolescents with symptomatic COVID-19 actually have a serious coagulopathy akin to adult patients (Table 3) [32]. Along with coagulopathy, an increase in D-dimer and elevated venous thromboembolism (VTE) rates were also observed in this group, especially those with severe respiratory complications. Based on data from one children’s hospital, the clinical features and laboratory results related to coagulation of 27 pediatric and young adult patients treated for symptomatic COVID-19 were investigated. A thromboembolism occurred. VTE occurred in 7 patients (26%), of whom 3 were DVT and 4 were pulmonary embolism.
Another report suggested that pediatric thromboprophylaxis may be helpful [33]. The basis for this suggestion is that children with multisystemic inflammatory syndrome can also have with coagulopathy. Children with multisystemic inflammatory syndrome (MIS-C) had a higher incidence of thrombotic events (6.5% [9 of 138]) than those with COVID-19 (2.1% [9 of 426]) or asymptomatic SARS-CoV-2 (0.7% [2 of 289]). Most thrombotic events (89%) occurred in COVID-19 or MIS-C patients who were 12 years of age or older.
Conclusion
The COVID-19 pandemic caused by SARS-CoV-2 has featured high morbidity and mortality rates. To overcome this, vaccines have been developed and administered worldwide at an unprecedented rate. However, unexpected adverse events have been reported but not yet been confirmed in clinical trials. In particular, thrombotic events after the SARS-CoV 2 vaccination, which has become an issue recently, have been accompanied by thrombocytopenia; therefore, it is currently being called VITT. Although its exact pathogenesis and mechanism are not yet known, consensus has been reached regarding VITT diagnosis and treatment. In addition, thrombocytopenia reportedly occurred after vaccination. Further data investigation and research are required to clarify the mechanisms and treatments for various vaccine-related issues.
Notes
No potential conflict of interest relevant to this article was reported.

