Growth patterns of preterm infants in Korea
Article information
Abstract
With advancements in neonatal care and nutrition, the postnatal growth of preterm infants has improved; however, it remains an issue. Accurate assessments of growth using a standardized reference are needed to interpret the intrauterine and postnatal growth patterns of preterm infants. Growth in the earlier periods of life can contribute to later outcomes, and the refinement of postnatal growth failure is needed to optimize outcomes. Catchup growth occurs mainly before discharge and until 24 months of age, and very low birth weight infants in Korea achieve retarded growth later in life. Knowing an infant’s perinatal history, reducing morbidity rates during admission, and performing regular monitoring after discharge are required. Preterm infants with a lower birth weight or who were small for gestational age are at increased risk of poor neurodevelopmental outcomes. Furthermore, poor postnatal growth is predictive of adverse neurodevelopmental outcomes. Careful monitoring and early intervention will contribute to better development outcomes and national public health improvements.
Key message
∙ The growth of preterm infants is a main focus of neonatology.
∙ Preterm infants in Korea, especially those with a very low birth weight, achieve retarded growth.
∙ Careful growth monitoring and early intervention will contribute to better development outcomes and quality of life for preterm infants and improve public health.
Graphical abstract. The incidence of poor growth (weight <10 percentile) in preterm infants. VON, Vermont Oxford Network; CPQCC, California Perinatal Quality Care Collaborative; KNN, Korean Neonatal Network; NHIS, national health insurance system in Korea; NICHD, National Institute of Child Health and Human Development.
Introduction
The recommendation to encourage preterm infants to grow in utero at a rate comparable to that of their age-matched peers is rather clear. However, their postnatal growth often does not achieve this goal. The percentage of infants with severe growth failure has decreased significantly over time. However, such improvements are insufficient. Various studies have examined the growth of preterm infants in Korea; however, few have focused on the data of preterm infants from a large-scale database. With advancements in neonatal care, the survival rates of premature infants in Korea have become comparable to those of medically advanced countries. Therefore, reviewing growth data for premature infants in Korea and comparing them with data from other countries can help improve the long-term growth and development and quality of life of premature infants in Korea.
Growth assessment
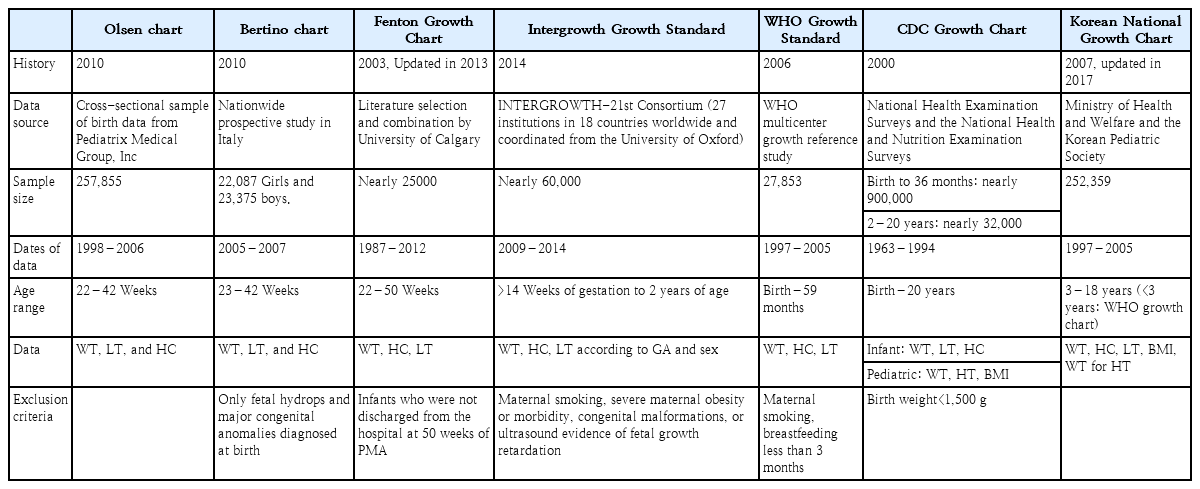
Neonatal growth charts that allow the calculation of precise z scores and percentiles were introduced in neonatology over the past decade. Growth standards are prescriptive and define how a population of children should grow if given optimal nutrition and health. Growth charts (references), on the other hand, describe a child’s growth at a certain point in time. Several charts and growth standards for interpreting intrauterine and postnatal growth patterns of infants have been introduced (Table 1), but their comparison is difficult due to age range variability and methodological problems.
Over 50 years ago, Lubchenco constructed the first intrauterine growth chart using birth records from the 1950s representing birth weight for gestational age at 24–42 weeks [1]. Olsen growth charts from the United States and Bertino growth charts from Italy assess appropriate for gestational age (AGA), small for gestational age (SGA), and large for gestational age status at a wide gestational age range of 22–23 to 42 weeks [2,3]. The Olsen growth chart included more diverse ethnicities [4]; however, it is limited because the sample included neonatal intensive care unit (NICU) admissions only, so they are known to perform relatively poor growth monitoring beyond 36 weeks of age.
The Fenton chart introduced fetal and infant growth reference data for 22–50 weeks in 2003 [5]. It was last updated in 2013 after a meta-analysis of 6 studies monitoring postnatal growth and revised to harmonize and continue using the World Health Organization (WHO) Growth Standard after 50weeks’ postmenstrual age [6]. However, it did not consider postnatal adaptations, especially by ignoring the physiological fluid loss that occurs in the first few days of life.
In 2014, the INTERGROWTH-21st Consortium published international postnatal growth standards for preterm infants 27–64 weeks’ postmenstrual age based on neonates with no major complications or risk factors or ultrasound evidence of fetal growth restrictions who were born to healthy mothers [7]. The INTERGROWTH-21st is a multicenter multiethnic population-based project that proposed growth patterns using a representative population sample from 8 geographical areas of the world. It aims to better adapt to premature growth of infants 24–33 weeks’ gestation. However, concerns remain that fetal growth parameters may differ among ethnicities and that population-based growth references for infants with the same genetic and environmental backgrounds should be used [8].
The Centers for Disease Control and Prevention growth charts for use in children from birth to 20 years were released in May 2000 based on the National Center for Health Statistics growth chart in 1977 with the addition of more recent comprehensive data and improvements in the statistical smoothing procedure [9]. These growth charts were based primarily on nationally representative survey data for healthy infants, excluded very low birth weight (VLBW) infants, were supplemented with limited data from other sources, and used a descriptive approach to generate a reference to describe the distribution of size for age in the United States.
In 2006, the WHO released international growth standards for children aged 0–59 months that are now accepted worldwide [10]. These standards include all children regardless of ethnicity and socioeconomic status and include those who were predominantly breastfed for at least 4 months and still breastfeeding at 12 months. However, the WHO growth charts, although recommended for preterm infants after term age, begin at term and do not assist preterm infant growth assessments prior to this age [6].
The Korean National Growth Chart (KNGC) was released in 2017 for the evaluation of body size and growth of Korean children and adolescents in private and public health clinics [11]. In children aged 0–35 months, a WHO growth chart was used with the recommendation of breastfeeding as the ideal nutritional supply for infants. For children and adolescents aged 3–18 years, height for age, weight for age, body mass index for age, weight for height, and head circumference (HC) for age charts were developed to overcome the limitations of the KNGC 2007 version.
In Korea, the Fenton growth chart is commonly used to assess preterm growth and promising for optimal preterm growth monitoring. After term-equivalent age is reached, the KNGC is widely used, although it is the same as the WHO chart for children younger than 3 years of age.
Postnatal growth during NICU admission
Among the 355 published preterm infant growth studies published since 2005, 19% showed extrauterine growth restriction (EUGR) or postnatal growth failure (PGF) rates mainly defined as being below the 10th percentile (62%) and the 3rd percentile (28%) at the time of discharge (61%), term (20%), or 36 weeks’ gestation (19%) [12]. According to previous reports of various neonatal networks, the rate of PGF among VLBW infants, defined as a discharge weight below the 10th percentile, was noted to exceed 95% in the 1990s in the National Institute of Child Health and Human Development (NICHD) study, a decreasing trend from 2000 to 2013 (65% to 50%) in the Vermont Oxford Network, and 53% using the Fenton growth chart in 2005–2012 from the California Perinatal Quality Care Collaborative (CPQCC) [13,14]. In a single-center study of data from the KNGC, 67% of VLBW infants were admitted between 2005 and 2009 versus 47% of VLBW infants admitted between 2010 and 2011 [15,16]. In the Korean Neonatal Network (KNN) study, the weight z score at discharge among surviving 2,799 VLBW infants was -1.37, showing a greater than 50% PGF incidence using the Fenton growth chart [17]. The incidence of PGF in Korea is comparable to those of other countries.
However, the term PGF requires refinement in the diagnostic criteria because EUGR is not predictive of adverse neurodevelopmental outcomes and is usually used to apply intrauterine growth restriction (IUGR) criteria [18]. The definition of malnutrition in preterm infants includes ow growth velocity (weight and length growth), change in weight z score, and previous nutrient intake. When PGF of VLBW was defined as a weight z score decrease from birth to discharge using the Fenton growth chart, the KNN reported that 45.5% of the infants had a score that exceeded 1.28, in accordance with the 41% of infants whose score exceeded 1.0 in the CPQCC [14,17]. Among the Israel National VLBW infant database, 8.1% and 35.5% had a decrease in z scores of >2 and 1–2 using a Canadian reference, respectively [19].
The growth of preterm infants is adversely affected by neonatal morbidities such as necrotizing enterocolitis, gastrointestinal perforations, intraventricular hemorrhage, retinopathy of prematurity, and bronchopulmonary dysplasia. In addition, nutrition-related factors such as enteral feeding and parenteral nutrition practices can be significant risk factors for PGF [20-22]. Infants born SGA or with IUGR had poor growth outcomes [17,23]. A growth assessment is necessary to elucidate how well an infant’s nutritional needs are being met and which infants have difficulty overcoming neonatal morbidities. Recent evidence points to the importance of true growth faltering for optimizing outcomes and supporting infants who are growing adequately to continue on their successful path rather than using a one-time size value at 40 weeks or prior.
It is challenging to further improve our understanding of the growth and nutritional needs of preterm infants during NICU admission. Furthermore, clinical judgment and individualization are needed to set growth goals and assess nutritional status to ensure better outcomes.
Postnatal growth after discharge, especially in VLBW infants
The incidence of growth failure after discharge is declining, but it has not yet been overcome. Table 2 presents a study of postnatal growth in premature infants after discharge. Research on VLBW infants using the KNGC at a single center in Korea revealed a 33% rate of weight below the 10th percentile at 24 months in 2000–2001, a 19% (18 of 98) rate of weight below the 10th percentile at 18 months of postchronological age (PCA) between 1996 and 2000, and a 12.4% (14 of 113) rate of weight below the 3rd percentile at 18 months of PCA between 1997 and 1999 [24-26]. A prospective cohort study of a total of 2,943 VLBW infants from the KNN between 2013 and 2015 showed a rate of 18.5% at 18–24 months of corrected age and a rate of 33.5% at 36 months of age for a weight z score <-1.28 using the WHO growth chart [27]. A nationwide study from the Korean National Health Insurance System (NHIS) database showed the rate of poor growth (defined as below the 10th percentile of weight) using the KNGC was 33% at 24 months, 26% at 36 months, 32% at 48 months, and 36% at 60 months of age [28]. Compared to our data, the incidence of growth under the 10th percentile at 1 year of age was 40.9% of 284 patients in China, while the NICHD in the United States indicated that 40% of patients still have a weight, length, and HC less than the 10th percentile at 18–22 months [13].

Characteristics of the studies included in the review of preterm infant growth after neonatal intensive care unit discharge
Although limited long-term data exist, many studies agree that, despite a period of catch-up growth, VLBW infants achieve retarded growth later in life [29]. A study in the Netherlands revealed that VLBW infants remain shorter and lighter at age 19 years than non-VLBW infants [30]. Accordingly, at age 20 years, former VLBW infants were twice as likely as normal birth weight infants to have a short stature below the 3rd percentile [31]. Studies also suggest that compromised growth may result in a different body composition and lead to adverse health outcomes later in life [32]. Thus, catch-up growth in VLBW infants should be monitored after discharge.
The weight growth pattern from birth to 36 months of age showed significant differences between SGA and non-SGA infants and between VLBW infants and extremely low birth weight (ELBW) infants in the KNN study [27]. Similarly, very preterm and SGA infants in Denmark showed a catch-up growth in weight and height at 6 years of age; however, they reached a significantly lower mean z score than AGA children [33].
Growth is influenced by various factors, including comorbidities and sex [9,21]. From the NHIS database, hyaline membrane disease, patent ductus arteriosus, intraventricular hemorrhage, sepsis, and bronchopulmonary dysplasia (BPD) significantly increased the risk of having a weight less than the 10th percentile of normal at 60 months of age [28]. In particular, infants with BPD reportedly showed an increased risk of growth failure after discharge of 30%–67%. This finding is consistent with the result as an odds ratio (OR) of 2.18 at 18–24 months from the KNN study and OR of 1.50 at 60 months from the NHIS study [27,28]. In a previous large-scale cohort study, infection in ELBW infants affected growth failure in weight and HC at both 36 weeks and 18–22 months of corrected age [26]. In Korea, infants with sepsis showed growth failure in weight (OR, 1.43) and HC (OR, 1.33) [28]. In accordance with a previous study, infants with periventricular leukomalacia (PVL) had a relatively small HC (OR, 1.91) at 60 months of age. Knowing an infant’s perinatal history, reducing the morbidity rate during admission, and performing regular check-ups after discharge can reduce the rate of growth failure.
Postnatal growth of preterm infants by preschool age, adolescence, and adulthood
Various studies have analyzed long-term growth patterns in premature infants using various definitions of poor growth and growth parameters (Table 2). Catch-up growth occurred mainly during the early neonatal period before 24 months and before discharge [33,34]; however, the gains did not always continue over time. In the NHIS study of 430,541 infants covering 99% of national births in 2013, significant differences were noted in height, weight, and HC, respectively, according to age at health check-up, birth weight group, and combination of age and birth weight [35]. The lower birth weight group showed lower catch-up growth, even at 60 months. The mean weight, height, and HC percentiles were persistently below 40 among low birth weight and VLBW infants [35]. The full-term group had greater heights than the preterm group at 8 years of age when the healthy preterm and mid-preterm groups caught up. A systematic review of growth patterns and body composition among ELBW neonates stated that, despite catch-up growth, their weight, height, and HC growths remained retarded into adulthood [29,36,37].
Pre- or full-term born SGA children were shorter and lighter until 3 years of age than AGA children [34,38-41]. The growth velocity of SGA children was significantly higher than that of AGA children in the first 2 years of life [39]. In a single-center study in Korea, 29% of SGA, 33% of severe SGA (<3rd percentile), and 36.4% of moderate SGA (3rd–10th percentile) infants did not reach catch-up growth for weight goals at 24 months of PCA [15,42]. In a Swedish population cohort study, SGA moderate preterm (32–35 weeks) infants were shorter and lighter throughout the 5-year follow-up and the association was stronger in SGA infants at birth [43]. The SGA group was still shorter and lighter at 12 years of age [40].
Nutrition is also a factor contributing to the growth of preterm infants; however, limited information is available on what it involves. Unfortified human milk-fed infants demonstrated slower catch-up growth that was extended until 6 years of age. Rapid weight growth was most pronounced shortly after discharge, especially in preterm formula-fed infants [33].
Growth and development
The correlations between growth and development revealed using various neurodevelopmental assessments in preterm infants are presented in Table 3. Although most studies focused on extremely preterm infants or VLBW infants [36,44,45], even moderate preterm (32+0–33+6 weeks’ gestational age) or late-preterm (34+0–36+6 weeks’ gestational age) infants were at increased risk of poor neurodevelopmental outcomes [46-48]. Being born preterm and SGA increased the risk of adverse developmental outcomes [49,50].
In accordance with other studies, from the NHIS study, infants with poor weight, height, and HC growth were at an increased risk of suspected developmental delay at 60 months of age. ELBW infants were more likely to have a suspected developmental delay (OR, 5.05) than infants with a birth weight of 2,500–4,500 g [35]. According to Korean retrospective cohort studies, HC at 4 months of PCA was related to favorable neurodevelopmental outcomes at 18 months of PCA, while an HC growth spurt between a postmenstrual age of 35 weeks and a PCA of 4 months is beneficial for preterm AGA infants [34]. Catch-up growth of body length was related to cognitive development, while catch-up growth of HC was related to both cognitive and motor development [51]. The period and indices differ between SGA and AGA infants, so they should be monitored differently [34]. Using multiple regression analysis to predict a risk factor for poor developmental outcomes at the age of 60 months, PVL (OR, 2.89), restricted HC growth (OR, 2.36), and restricted height growth (OR, 2.42) were confirmed [28].
Growth in the early postnatal period affects neurodevelopmental outcomes at school age and adolescence [36,52]. Even during adulthood, psychological and behavioral problems are increased in the poor growth group [53], and all height, weight, and HC growth parameters are related to developmental outcomes [54]; however, the association between HC and neurodevelopment was more closely examined [34,45,51,55,56]. Brain size at term-equivalent age was associated with brain tissue volumes on magnetic resonance imaging [45]. Poor postnatal head growth in preterm infants becomes more evident by 2 years and is strongly associated with poor neurodevelopmental outcomes and cerebral palsy. Earlier catch-up HC growth was more critical for neurodevelopment than catch-up weight and length growth [51].
In a Japanese population study, SGA infants with poor postnatal growth were at risk for attention problems throughout preschool to school age. Twenty-six percent of preterm SGA infants failed to catch up. SGA children lacking catch-up growth were more likely to be unable to listen without fidgeting (OR, 2.51) and unable to focus on a task (OR, 2.66) than non-SGA children at 5.5 years of age. Furthermore, SGA children lacking catch-up growth were at a significant risk of inattention at 8 years of age. In contrast, the SGA and catch-up groups were not at increased risk of attention problems or delinquent/aggressive behaviors [57]. In a Brazilian cohort study, preterm infants born with IUGR were at increased risk of motor delays [58]. Thus, children born preterm and SGA, especially those lacking catch-up growth, should be monitored cautiously.
Conclusion
Recent improvements in preterm survival have shifted the focus on preterm infants toward improving their quality of life by monitoring their growth and development. In Korea, preterm infants still show differences in growth and development until preschool age. Thus, careful monitoring and early intervention are required.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.