Upper gastrointestinal tract involvement of Crohn disease: clinical implications in children and adolescents
Article information
Abstract
Crohn disease (CD) is a multifactorial inflammatory disorder that can affect all segments of the gastrointestinal (GI) tract but typically involves the ileum and/or colon. To assess patient prognosis and choose appropriate treatment, it is necessary to accurately evaluate the factors influencing poor outcomes, including disease phenotype. Pediatric CD involving the upper GI (UGI) tract has become increasingly recognized with the introduction of routine upper endoscopy with biopsies for all patients and the increased availability of accurate small bowel evaluations. Most clinical manifestations are mild and nonspecific; however, UGI involvement should not be overlooked since it can cause serious complications. Although controversy persists about the definition of upper GI involvement, aphthoid ulcers, longitudinal ulcers, a bamboo joint-like appearance, stenosis, and fistula are endoscopic findings suggestive of CD. In addition, the primary histological findings, such as focally enhanced gastritis and noncaseating granulomas, are highly suggestive of CD. The association between UGI involvement and poor prognosis of CD remains controversial. However, the unstandardized definition and absence of a validated tool for evaluating disease severity complicate the objective assessment of UGI involvement in CD. Therefore, more prospective studies are needed to provide further insight into the standardized assessment of UGI involvement and long-term prognosis of CD. Our review summarizes the findings to date in the literature as well as UGI involvement in CD and its clinical implications.
Key message
· Clinical manifestations of upper gastrointestinal (UGI) tract involvement in Crohn disease (CD) are common but often clinically underestimated.
· Diagnosing CD by confirming inflammation of the UGI tract histologically is challenging because macroscopic and microscopic findings overlap with those of other diseases.
· Ongoing efforts are needed to enable a standardized assessment of UGI CD in the future.
Graphical abstract. Summary of upper gastrointestinal involvement of Crohn disease
Introduction
Crohn disease (CD) is a multifactorial and inflammatory disorder of the gastrointestinal (GI) tract characterized by a relapsing-remitting clinical course with the progressive accumulation of bowel damage [1]. Approximately 25% of patients with CD are diagnosed during childhood and adolescence [2]. The incidence and prevalence of CD have increased remarkably, indicating its emergence as a global disease [3]. Especially in pediatric patients, the incidence of CD increased from 5.2 per 100,000 (95% confidence interval [CI], 4.3–6.2) in 1994 to 7.9% per 100,000 (95% CI, 6.9–9.0) in 2009 (P<0.0001) [4]. Similarly, the incidence of pediatric CD has increased exponentially in South Korea [5].
To assess the prognosis and implement appropriate treatment for patients with CD, it is necessary to accurately evaluate the factors influencing poor outcomes. The factors affecting the disease course of CD are disease phenotypes including young age at diagnosis, early stricturing (B2) and/or penetrating (B3) disease behavior, perianal disease, and upper GI (UGI) involvement with/without extensive small bowel disease [6-8]. To facilitate clear disease phenotyping, inflammatory bowel disease (IBD) experts categorize CD into 4 phenotypes according to disease location: ileal (L1), colonic (L2), ileocolic (L3), and UGI (L4) disease, that is, proximal CD, according to Montreal classification for adults and Paris classification for pediatric patients [9,10]. Pediatric CD involving the UGI tract has become increasingly recognized by the introduction of routine upper endoscopy with biopsies for all patients being evaluated for CD [11]. The recently increased availability of accurate small bowel evaluations such as capsule endoscopy and magnetic resonance enterography (MRE) as well as computed tomography (CT) has led to increased reporting of the UGI involvement of CD [12].
It is generally known that UGI involvement is more prevalent in pediatric than in adult CD patients and that a younger age at diagnosis in both adults and children is associated with UGI involvement [13]. In addition, a male sex predominance is reported at a ratio of 1.2:1 for UGI involvement among patients with CD [14]. The mean age of pediatric patients diagnosed with proximal CD is 10.9 years compared with 12.6 years for those diagnosed with distal CD [15]. For adult patients with CD, the mean age at diagnosis is 21.2 years for proximal disease versus 25.4 years for distal disease [16]. This review discusses the UGI involvement of CD, one of its phenotypes that is known to affect prognosis.
Definitions of oral and UGI involvement of CD
Oral involvement is frequently reported in CD and is usually called oral CD (OCD) [17]. The definition of OCD is highly variable, including lip swelling, cobble stoning of the buccal mucosa, ulceration and fissuring of the oral cavity, and/or gingival swelling [18]. Regardless, histological confirmation is required in all cases of OCD.
Although controversy persists about the definition of UGI involvement in CD, it is generally defined as mucosal ulcerations of the UGI tract on endoscopy or bowel wall thickening on radiography [10]. The presence of mucosal erythema and/or granularity is insufficient to be considered evidence of UGI involvement. The generally accepted diagnostic criteria for UGI involvement of CD were proposed by Nugent and Roy: (1) characteristic histology with noncaseating granuloma, with or without obvious CD elsewhere in the intestinal tract in the absence of a systemic granulomatous disorder, or (2) documented CD elsewhere in the intestinal tract and radiological or endoscopic findings of diffuse inflammation in the UGI tract consistent with CD [19].
The UGI involvement of CD is categorized by the Montreal and Paris classifications as an L4 phenotype, and L4 can be added to L1–3 classification when concomitant UGI disease is present [9,10]. The L4 phenotype is defined as upper disease from the esophagus to the proximal 2/3 of the ileum. L4 disease is further divided into upper disease proximal to the ligament of Treitz (L4a), upper disease distal to the ligament of Treitz and proximal to distal 1/3 ileum (L4b), or both (L4ab) according to the Paris classification [10]. The disease’s location should be defined by macroscopic rather than histological findings in normal-appearing mucosa.
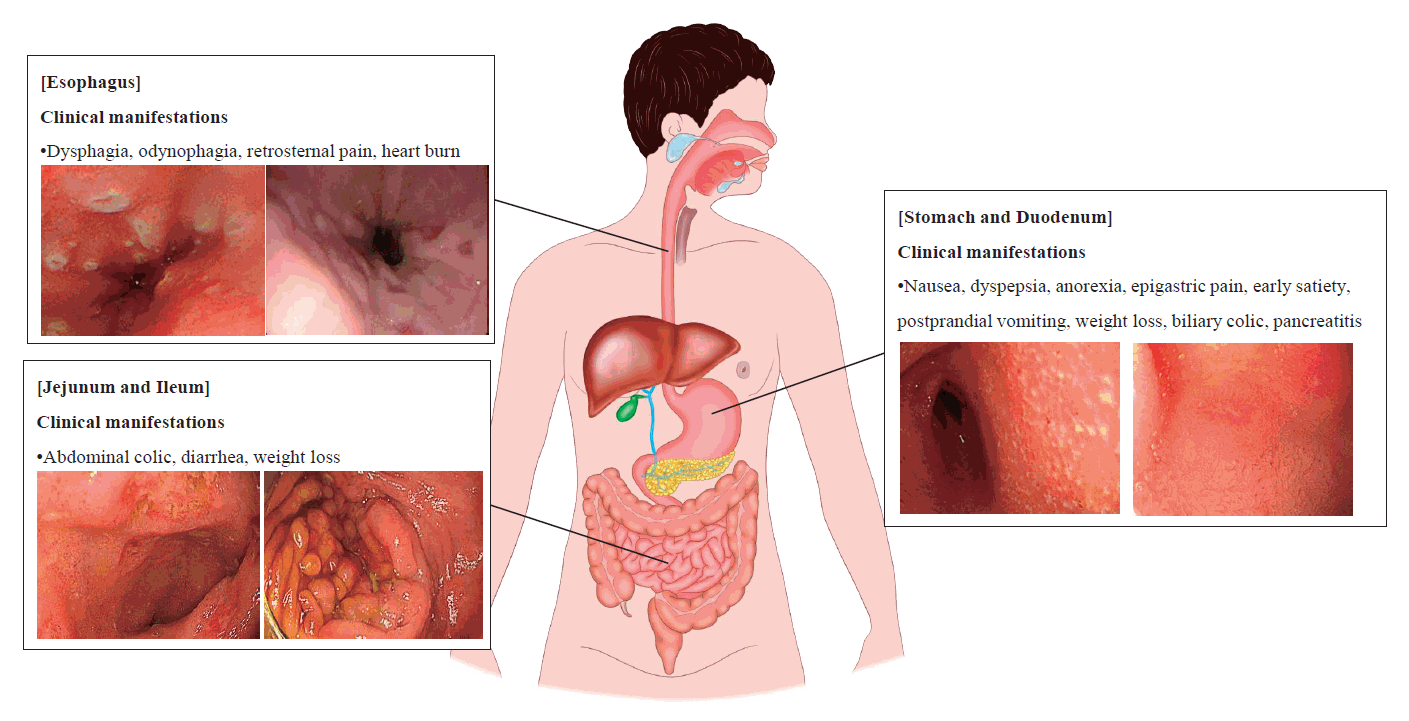
Clinical manifestations of UGI involvement of CD
UGI symptoms precede distal GI symptoms in only 10% of patients with CD, meaning that the L4 phenotype has a long asymptomatic phase [20]. Therefore, UGI involvement is underestimated and less commonly reported than lower GI involvement and usually occurs in patients with established ileal, large intestinal, or perianal CD. Nevertheless, the UGI involvement of CD should not be overlooked, as it can cause serious complications such as gastric outlet obstruction [21]. Clinical manifestations of UGI tract involvement of CD vary by location.
The esophageal involvement of CD was initially described by Franklin and Taylor in 1950 [22]. Most clinical manifestations of esophageal involvement are asymptomatic or mild with nonspecific UGI symptoms similar to gastroesophageal reflux disease; however, symptoms such as dysphagia, odynophagia, retrosternal pain, or severe heartburn may also occur [14]. In 2012, esophageal CD was hypothesized to progress through 3 phases [23]. The first phase involves inflammation, erosions, edema, and linear ulcers, followed by stenotic lesions with mucosal bridges (second phase). In the third stage, odynophagia, vomiting, and weight loss occur due to esophageal stricture.
Symptomatic gastroduodenal manifestations of CD occur in less than 4% of patients with CD [24]. Nonspecific symptoms such as nausea, dyspepsia, anorexia, and epigastric pain are the most frequent symptoms of gastroduodenal CD. Serious complications such as gastric outlet obstruction can cause early satiety, postprandial vomiting, weight loss, and rarely hematemesis [25]. In rare cases, biliary colic may occur secondary to the involvement of the ampulla of Vater [26] or pancreatitis due to duodenal involvement [27,28].
If the lesion is confined to the jejunum or proximal ileum, abdominal colic is the main presenting symptom. Other GI manifestations such as diarrhea, weight loss, and fever are also present, and abnormal laboratory results, including anemia, hypoalbuminemia, iron deficiency, and folic acid deficiency, also occur in the majority of patients [29].
Endoscopic findings of UGI involvement of CD
According to previous studies, the frequency of esophageal involvement in patients with CD is 0.2%–6.0%, lower than that in the stomach and duoenum [30-32]. The macroscopic findings of esophageal lesions range from scattered erosions to ulcers that frequently have a longitudinal tendency (Fig. 1A) to those with a cobblestone appearance, and to more severe forms such as fistula and stricture [12,33-35]. However, these lesions are not specific to CD, so eosinophilic esophagitis, viral and fungal infections, tuberculosis, vasculitis, and malignancies must be excluded [36]. Most esophageal lesions are located in the middle or distal esophagus or appear as diffuse inflammation on esophagogastroduodenoscopy (EGD) [34].

Upper endoscopic findings of Crohn disease. (A) Esophageal ulcers showed a longitudinal tendency in Crohn disease. (B) Gastric ulcers and erosions in Crohn disease. (C) Duodenal ulcers in Crohn disease.
Compared to esophageal involvement, gastric and duodenal involvement are relatively frequent findings in CD [18,36]. However, before the routine use of EGD to diagnose CD, gastroduodenal lesions were considered rare, occurring in an estimated 0.5%–4% of patients with CD [37]. At that time, most studies did not explain the specific endoscopic findings of proximal CD, and it was believed that only the identification of noncaseating granulomas on a histopathological examination confirmed the diagnosis [38,39]. One of the most significant studies of the upper endoscopic findings of CD was reported in 1997 [40]. The authors reported that a bamboo joint-like appearance on EGD is a characteristic endoscopic finding in CD. Since then, many studies have attempted to identify the characteristic upper endoscopic findings of CD and the diagnostic value of EGD [41-44].
EGD with biopsy is the gold standard for the diagnosis of UGI CD. Endoscopic findings of gastroduodenal CD include ulceration, erosions, patchy erythematous mucosa, thickened folds, cobblestone appearance, bamboo joint-like appearance, fistula, and strictures [12,45]. Erosions often appear irregular and longitudinal, while ulcers can have various shapes such as aphthoid, linear, serpiginous, or stellate (Fig. 1B, C) [37,45-47].
As mentioned above, longitudinal ulcers, fistulae, and strictures of the UGI tract are considered endoscopic findings specific to CD regardless of location. Although most macroscopic findings in EGD are nonspecific for CD, some endoscopic findings may be characteristic when at specific locations in the UGI tract. The findings specific to UGI CD include aphthae, erosions, and ulcers in the esophagus [46]; a bamboo joint-like appearance in the stomach [40,41]; a notch-like appearance and nodular folds in the duodenum [42]; and nodular lymphoid hyperplasia, a villous pattern, and a cobblestone appearance in the small bowel [48].
The incidence of UGI involvement is 30%–64% when routine EGD is performed in patients with CD [15,49]. The EUROKIDS and the Hungarian IBD Registry reported that 35%–67% of pediatric patients with CD showed endoscopic abnormalities of the UGI tract and 9%–24% demonstrated characteristic macroscopic findings of CD [11,43]. This variation is largely associated with the lack of a standardized definition of UGI CD [44].
An Israeli group recently proposed a formalized scoring system for the UGI involvement of CD, the UGI-Simple Endoscopic Score for CD (UGI-SES-CD), which correlates with disease severity [50]. The UGI-SES-CD uses the same criteria as the SES-CD, a scoring system that evaluates the severity of the lower GI tract: ulcer size, ulcerated surface, affected surface, and narrowing to assess UGI CD severity. Disease severity is evaluated as the sum of the scores of the 4 regions (esophagus, stomach, antrum, and duodenum). As reported in that study, ongoing efforts are needed to enable a standardized assessment of UGI CD in the future.
Pathologic findings of UGI involvement of CD
Diagnosing CD by confirming inflammation of the UGI tract on a histological examination is challenging because its macroscopic and microscopic findings overlap with those of other diseases such as gastroesophageal reflux disease, immune-mediated disorders, and Helicobacter pylori infection. However, the histologic examination of the UGI tract is useful in diagnosing patients with IBD-unspecified or unclassified diagnoses, especially pediatric patients [44].
Esophageal involvement in patients with CD can be histologically seen in the form of active esophagitis, chronic esophagitis, and reflux esophagitis [51-53]. Inflammation is usually nonspecific, including erosions, ulcers, granulation tissue, or lymphoplasmacytic infiltrates. Lymphocytic esophagitis occurs in 12%–28% of pediatric CD patients but it is not a characteristic finding in adults [51,54].
Focally enhanced gastritis, chronic active and inactive gastritis, and chronic atrophic gastritis are nonspecific inflammatory patterns that are found in the stomach [38,45,55], while duodenal inflammation is characterized by increased intraepithelial lymphocytes, crypt inflammation, and villous blunting [56].
Focally enhanced gastritis, which consists of focal collections of histiocytes and lymphocytes surrounded by gastric foveola (Fig. 2A), is found in CD patients with a prevalence of 43%–76% [55,57,58]. The reported sensitivity and specificity of focally enhanced gastritis for IBD are 35.7% and 96.6%, respectively [59,60]. The presence of focally enhanced gastritis is highly associated with IBD in pediatric patients; however, it does not reliably distinguish between CD and ulcerative colitis.
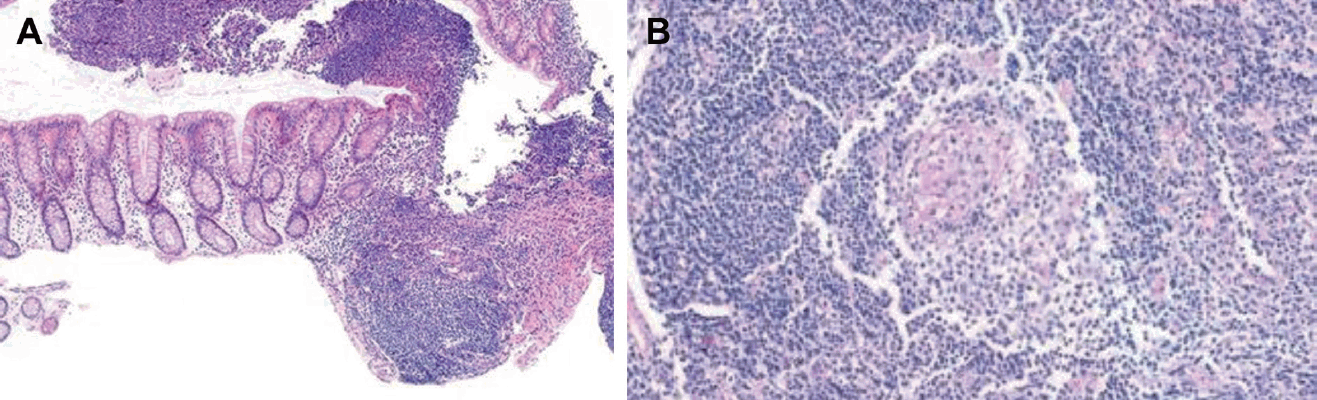
Histopathologic findings of upper gastrointestinal tract of Crohn disease. (A) Gastric biopsy specimens showing small clusters of lymphocytes in the lamina propria indicating focally enhanced gastritis. (H&E, ×4) (B) Duodenal biopsy specimens showing granuloma. (H&E, ×64)
H. pylori, one of the leading causes of gastritis, mimics the features of UGI involvement of CD on histologic examination. According to the Second Asia-Pacific Consensus Guidelines for H. pylori infection, H. pylori infection should be excluded before the diagnosis of UGI CD can be made [61]. The H. pylori infection rate among CD patients is reportedly low [62-66]. Moreover, Park et al. [67] reported that H. pylori infections are relatively uncommon in Korean pediatric patients with CD.
Noncaseating granulomas are a specific histological finding of CD (Fig. 2B), but they are more difficult to detect in biopsy samples than in surgical specimens [68]. Granulomas can also be found in focal lesions or macroscopically in the normal mucosa [20]. Granulomas are most often identified in the gastric antrum (25%), followed by the duodenal bulb (11%) and the proximal or middle stomach (6%) [45,57]. Although the overall prevalence depends not only on biopsy number, depth, and quality, it also depends on the location of the affected UGI tract; thus, granulomas are found in 11%–40% of patients with CD [45].
There is increasing interest in the endoscopic and histological differences by CD extent. Histological disease is reportedly more extensive than endoscopic disease at the time of CD diagnosis [69]. Although mucosal healing is a treatment target in the management of CD [70], validated tools for the classification of CD, such as the Paris classification, have no histological considerations. Therefore, the development of a classification system that divides disease extent using both endoscopic and histological criteria is necessary.
Prognosis of UGI involvement of CD
It is generally known that UGI involvement, that is, the L4 phenotype, in CD patients predicts a more severe disease phenotype requiring more aggressive treatment than those without UGI involvement [71]. L4 disease is associated with more extensive disease and extraintestinal manifestations [15]. However, the association between UGI involvement and a poor prognosis of CD remains controversial (Table 1).
Some studies argued that there is no significant relationship between L4 disease and CD prognosis [15,30,36]. Crocco et al. [15] reported no difference in prognosis between pediatric patients with and those without L4 disease. An analysis of Korean pediatric patients revealed no intergroup differences in complications requiring intestinal resection [30,72], and the same result was obtained in a subgroup analysis of the presence or absence of jejunal involvement [30]. However, in both studies, patients with UGI involvement required more aggressive treatment than those without such involvement.
Other studies reported that UGI involvement is associated with poor outcomes such as relapse and the need for surgery [15,73-75]. Chow et al. [76] revealed that a Chinese cohort of patients with UGI involvement had a more severe disease course, including strictures, fistulae, and the risk of longer hospitalization. Another study of a Chinese cohort reported that patients with L4 disease and UGI involvement showed higher rates of abdominal surgery (L4 disease vs. non-L4 disease: 41.3% vs. 11.4%) but similar rates of hospitalization [73].
Other recent studies indicated that only L4-jejunal and L4-proximal ileal phenotypes, rather than all UGI phenotypes, are associated with a higher risk of a poor prognosis [16,75,77,78]. Kim et al. [75] found that the surgery-free survival rate of patients with proximal small bowel involvement was lower than that of patients without such involvement (58.4% vs. 67.7%). In addition, Lazarev et al. [16] proposed that the L4 phenotype is heterogeneous in terms of disease phenotypes and outcomes. One study reported that patients with L4-jejunal disease had more strictures and fistulae requiring abdominal surgeries than those with L4-esophagogastroduodenal disease.
Comparison of Korean and European patients
Most studies to date reporting on UGI involvement in patients with CD are retrospective cohort studies. The prevalence of UGI tract involvement in adults is approximately 0.5%–16%, and the diagnostic rate is increasing with the use of recently developed diagnostic techniques [15,16,73]. According to studies conducted in a European pediatric cohort, the prevalence of UGI involvement is 46.2% [49]. However, UGI involvement in Korean pediatric patients is reportedly 50.0%–74.4%, higher than that of European patients [29,67]. Kim et al. [30] reported that, compared to the EUROKIDS registry, Korean pediatric patients had significantly higher UGI involvement rates than European pediatric patients (74.4% vs. 46.2%, P<0.001). Despite the use of the same strict definition of UGI tract involvement, the prevalence of UGI involvement differs between Korean and European pediatric patients.
One possible explanation for this difference is that race may play an important role in the expression of different phenotypes in pediatric CD. Another explanation is the differences in the modalities of small bowel evaluation (L4b phenotype). The study of Korean pediatric patients was conducted from 2004 to 2019, and all patients underwent an accurate small bowel evaluation including MRE or capsule endoscopy [29]. However, since the EUROKIDS study was conducted from 2004 to 2009, less than half of the patients received advanced modalities for the small bowel evaluation. A total of 64% of patients were evaluated with small bowel follow-through, 38% with MRE, 6% with CT, and 5% with capsule endoscopy [49]. In fact, jejunal/ileal disease (L4b or L4ab) was identified in 48.1% of Korean pediatric patients compared to 24.1% of European pediatric patients. Further studies using the same definitions and evaluation methods are needed to enable accurate comparisons.
Conclusion
Pediatric CD involving the UGI tract has become increasingly recognized by the introduction of routine upper endoscopy with biopsy for all patients and the increased availability of accurate small bowel evaluations. Defining the disease phenotype helps clinicians assess disease severity and choose appropriate treatment strategies. Therefore, clinicians should be aware of the clinical, endoscopic, and histopathological findings of UGI involvement in CD. Debate persists about the association between UGI involvement in CD and disease prognosis. However, the lack of a standardized definition and absence of a validated tool for evaluating disease severity complicate the objective assessment of UGI involvement in CD. Therefore, future prospective studies are needed to provide further insight into the standardized assessment of UGI involvement in CD and determine long-term prognosis.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.