Viral load and rebound in children with coronavirus disease 2019 during the first outbreak in Daegu city
Article information
Abstract
Background
Viral load and shedding duration are highly associated with the transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. However, limited studies have reported on viral load or shedding in children and adolescents infected with sudden acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Purpose
This study aimed to investigate the natural course of viral load in asymptomatic or mild pediatric cases.
Methods
Thirty-one children (<18 years) with confirmed SARS-CoV-2 infection were hospitalized and enrolled in this study. Viral loads were evaluated in nasopharyngeal swab samples using real-time reverse transcription polymerase chain reaction (E, RdRp, N genes). cycle threshold (Ct) values were measured when patients met the clinical criteria to be released from quarantine.
Results
The mean age of the patients was 9.8 years, 18 (58%) had mild disease, and 13 (42%) were asymptomatic. Most children were infected by adult family members, most commonly by their mothers. The most common symptoms were fever and sputum (26%), followed by cough and runny nose. Nine patients (29%) had a high or intermediate viral load (Ct value≤30) when they had no clinical symptoms. Viral load showed no difference between symptomatic and asymptomatic patients. Viral rebounds were found in 15 cases (48%), which contributed to prolonged viral detection. The mean duration of viral detection was 25.6 days. Viral loads were significantly lower in patients with viral rebounds than in those with no rebound (E, P=0.003; RdRp, P=0.01; N, P=0.02).
Conclusion
Our study showed that many pediatric patients with coronavirus disease 2019 (COVID-19) experienced viral rebound and showed viral detection for more than 3 weeks. Further studies are needed to investigate the relationship between viral rebound and infectiousness in COVID-19.
Key message
Question: What is the natural course of viral load in children with coronavirus disease 2019 (COVID-19)?
Finding: A significant number of patients still had a relatively high viral load once clinically asymptomatic. Nearly half of the patients experienced viral rebound, which contributed to prolonged viral detection in their respiratory specimens.
Meaning: Further studies are needed to determine the clinical significance of viral rebound in asymptomatic or mild pediatric cases of COVID-19.
Graphical abstract. COVID-19, coronavirus disease 2019; RT-PCR, real-time transcription polymerase chain reaction; E, envelope; RdRp, RNA-dependent RNA polymerase; N, nucleocapsid; Ct, cycle threshold.
Introduction
In the past 2 decades, 2 epidemic human coronaviruses, severe acute respiratory syndrome coronavirus (SARS-CoV) and middle east respiratory syndrome coronavirus caused serious outbreaks [1]. In 2019, a novel coronavirus (SARS-CoV-2) was identified as the causative agent of pandemic coronavirus disease 2019 (COVID-19) [2]. So far, published data have reported that COVID-19 shows diverse disease severity according to the age and underlying disease of the patients [3-6]. Infection rate of COVID-19 in children is known to be relatively low [3,7] and a recent report showed that most pediatric cases were mild or asymptomatic [8]. The transmission of SARS-CoV-2 in children primarily occurs within families, but mild or asymptomatic pediatric cases might be important transmitters in the community [6]. Viral load in the respiratory tract samples and duration of viral detection are highly associated with the transmission of viral infection [9]. However, little is known about the viral load or duration of viral detection in the children and adolescents infected with SARS-CoV-2.
In Korea, the nation’s first outbreak of COVID-19 started in Daegu city from mid-February, 2020. The present study is the first summarized report of the children hospitalized with COVID-19 in Daegu city. We aimed to investigate the viral load and viral detection in our pediatric patients who were asymptomatic or had mild disease. We also examined the relationship between viral load or viral rebound and clinical characteristics of the patients.
Methods
1. Subjects
The present study enrolled 31 children with COVID-19, under 18 years of age, who were admitted to 3 hospitals in Daegu city, Korea, between February 18 and April 30, 2020. Daegu city was the place where the nation's outbreak of COVID-19 began and the highest infection rate was recorded in Korea.
According to the government’s quarantine measures, the children who were confirmed to be infected with SARS-CoV-2 by real-time transcription polymerase chain reaction (RT-PCR) tests which were performed at COVID-19 screening stations were hospitalized and isolated. The medical records of the patients were retrospectively reviewed to investigate the clinical and laboratory findings. Loss of smell or taste was evaluated with a questionnaire reported by the parents or patients themselves using snacks or food products.
Informed written consents were obtained from all parents and also from adolescent patients. This study was approved by Institutional Review Board (IRB) of Daegu Catholic University Medical Center (IRB No. CR-20-071) and Daegu Fatima Hospital (DEF 20 ORIO 066).
2. Virologic examination
According to Korean Central Disease Control Headquarters guidelines of COVID-19 (the 7th edition, March 15, 2020), the first RT-PCR tests after admission were performed when the patients met the clinical criteria to be released from quarantine, at least 7 days after symptom onset with the absence of clinical symptoms and radiological findings (Fig. 1).
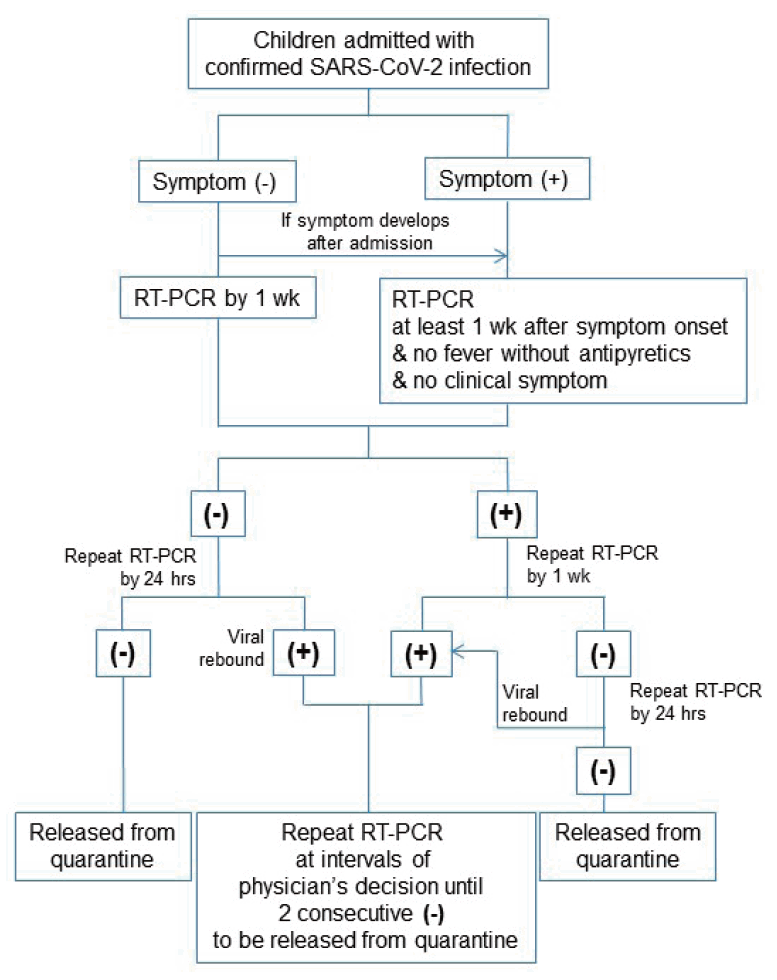
Flow sheet algorithm used for follow-up of SARS-CoV-2 RT-PCR tests after admission according to Korean Central Disease Control Headquarters coronavirus disease 2019 guidelines. SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; RT-PCR, real-time transcription polymerase chain reaction.
Nasopharyngeal swab samples were taken through a nostril by trained medical doctors. Qualitative RT-PCR assays were performed for the detection of 3 target genes of SARS-CoV-2; envelope (E) gene, RNA-dependent RNA polymerase (RdRp) gene, and nucleocapsid (N) gene (Allplex 2019-nCoV Assay; Seegene, Seoul, Korea) [10]. The results were considered negative only when all 3 target gene assays were negative. When any of them was positive, the results were considered positive.
Viral load was expressed by cycle threshold (Ct) value of RT-PCR test, a semiquantitative measurement of viral load [11], which is inversely correlated with viral load. In this study, we measured the Ct values of the first RT-PCR tests performed after admission when the patients met the clinical criteria to be released from quarantine. The Ct values of initial RT-PCR tests at the time of diagnosis before admission were unavailable in this study. Ct value of each target gene less than 40 was defined as a positive result. The viral load was categorized into 3 groups; high (Ct value; ≤20), intermediate (Ct value; 20–30), and low (Ct value; >30) [11].
Viral rebound was defined as repeated detection of SARS-CoV-2 genes despite previously shown negative RT-PCR tests taken 24 hours apart (Fig. 1). We investigated the viral load associated with viral rebound during the course of COVID-19. The duration of viral shedding was assessed based on the duration of viral detection in serial nasopharyngeal swab samples. In our study, duration of viral detection was defined as the time interval between the initial positive RT-PCR tests for diagnosis of COVID-19 and the first day of 2 consecutive negative RT-PCR tests taken 24 hours apart.
3. Statistical analysis
All statistical analyses were performed using MedCalc for Windows, version 19.4.0 (MedCalc Software, Ostend, Belgium) and R version 3.6.0 software (http://www.r-project.org, R Foundation for Statistical Computing, Vienna, Austria). The Fisher exact test was used to compare categorical variables between the 2 groups. For comparison of Ct values and duration of viral detection between 2 patient groups, since not all distributions were normal, Mann-Whitney U test was used. The differences of Ct values among the 3 target genes or patients’ subgroups stratified by age and symptoms were estimated with the Kruskal-Wallis test. A P value less than 0.05 was considered statistically significant.
Results
1. Demographic and clinical characteristics
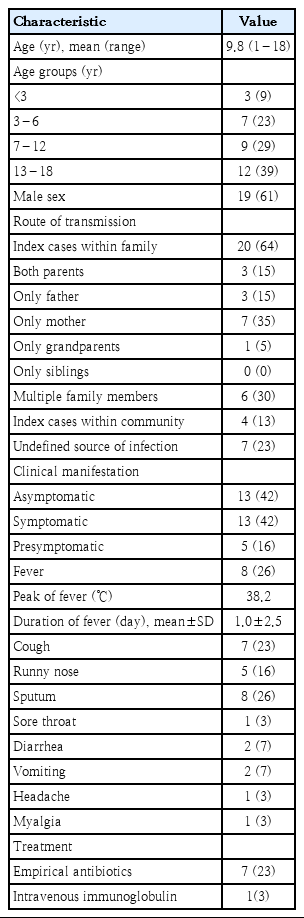
The demographic and clinical characteristics of the patients are shown in Table 1. Mean age of our patients was 9.8 years. The proportion of children under 3 years of age tended to be lower compared with other age groups (P=0.12).
In 24 children (77%), the transmission route of SARS-CoV-2 infection was identified. Twenty cases were infected from contact with family members, and the proportion was high when only mother was infected or multiple family members were infected. In 7 children (23%) who were tested because of mild upper respiratory infection (URI) symptoms and diagnosed with COVID-19, the route of transmission was not identified.
Among 31 patients, 18 (58%) showed clinical symptoms and 13 (42%) were asymptomatic. Symptomatic patients were classified into 2 groups, symptomatic group (N=13) and presymptomatic group (N=5), depending on whether the patients had symptoms at the time of diagnosis or subsequently developed symptoms. The mean interval between symptom onset and diagnosis was 7.2 days in symptomatic group. In presymptomatic group, the symptoms developed after diagnosis with a mean interval of 12.8 days.
None of our patients had dyspnea or other severe respiratory symptoms. Loss of smell or taste was not observed in our patients: 28 children over age 3 could answer to the questionnaire and the parents reported for 3 children under age 3 (17 months, 18, months, and 28 months of age). Four patients (13%) were diagnosed as having mild pneumonia by radiologic examination. Two of them had mild URI symptoms, 1 patient had only fever, and 1 patient was asymptomatic. Three patients (10%) had underlying medical conditions such as recurrent febrile seizure, chronic otitis media, and congenital hydronephrosis.
Empirical antibiotics were used in 4 patients with mild pneumonia, 1 with pre-existing otitis media, and 2 with cough. One patient with mild pneumonia who had prolonged mild fever (37.6°C–38°C) for 12 days without respiratory symptoms was given intravenous immunoglobulin (IVIG, 0.5g/kg, for 2 days). None of our patients needed oxygen supplement or treatment with any antiviral agents.
2. Laboratory and radiologic findings
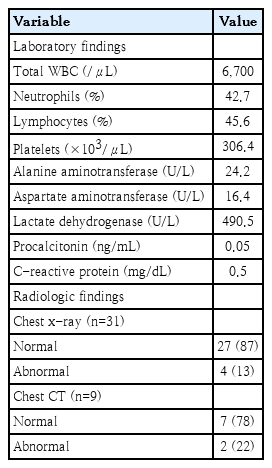
The results of routine hematologic and clinical chemistry tests showed normal values and presented in Table 2.
Chest x-rays were taken from all patients, and 4 cases (13%) showing slightly increased pulmonary markings were diagnosed with mild bronchopneumonia. High-resolution computed tomography scans were performed in 9 patients (29%), and 2 of them showed mild abnormal findings: mild bronchopneumonia in 1 patient (also shown in chest x-ray) and a small peripheral pulmonary nodule in the other one.
3. Viral load and viral rebound during SARS-CoV-2 infection
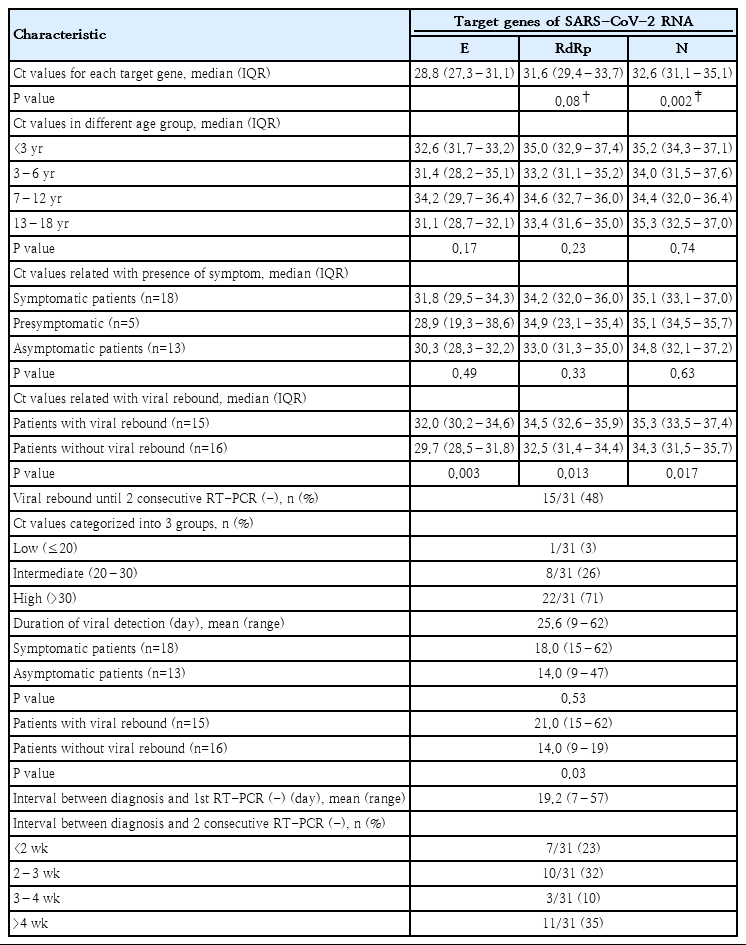
Ct values of E gene were significantly lower than those of N gene (P=0.002) and tended to be lower than those of RdRp gene (P=0.08). There was no significant difference in the Ct values of each target gene among different age groups (Table 3).
Ct values showed no difference among the symptomatic, presymptomatic, and asymptomatic patients (Table 3).
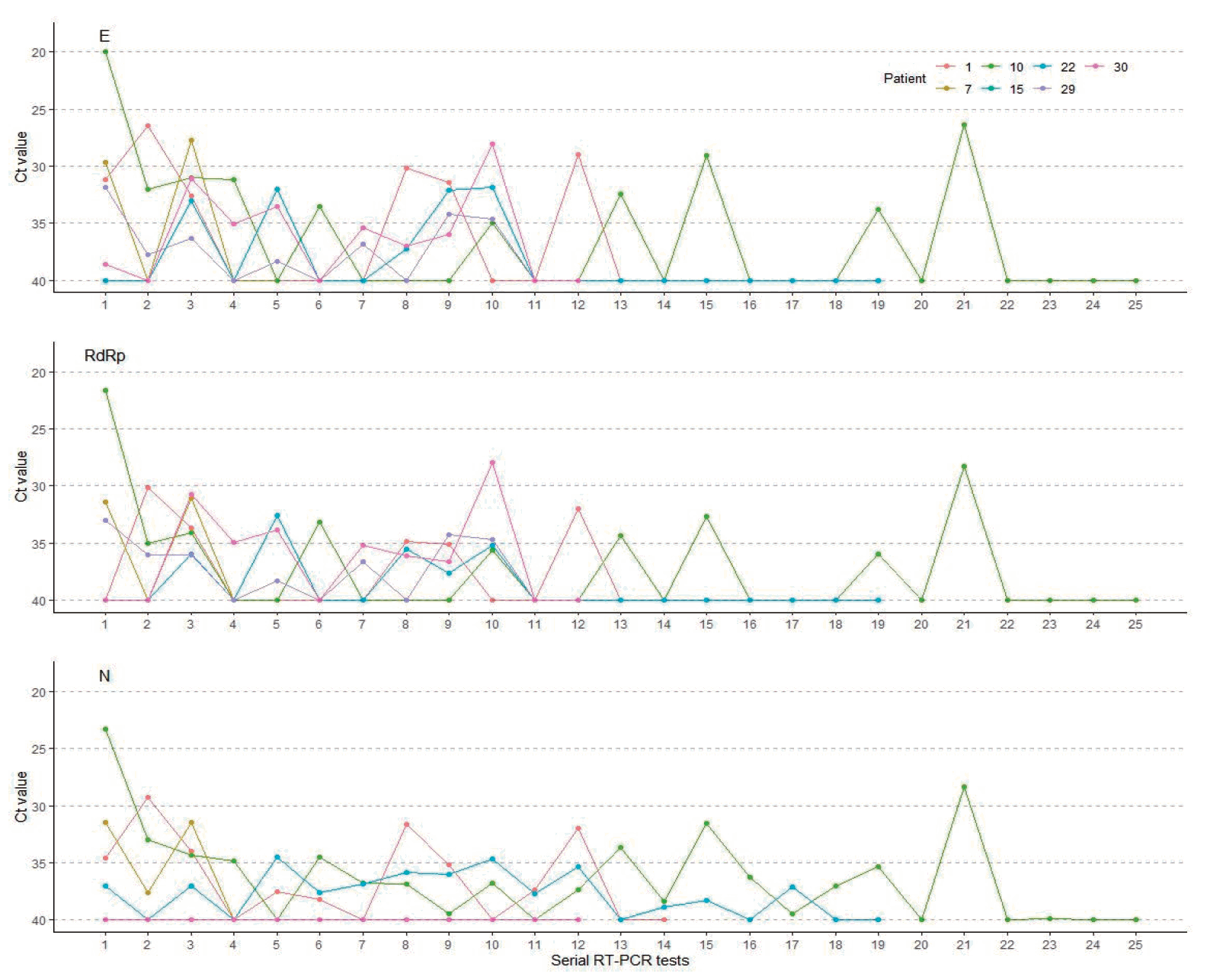
Viral rebound was found in 15 cases (48%), and 8 of them showed multiple rebounds. A total of 212 samples of 31 patients were tested and the average number of the tests performed per patient was 7 times, ranged from 2 to 25 times, until the patients received 2 consecutive negative RT-PCR test results. One patient who received IVIG treatment showed no viral rebound. Ct values of 3 target genes were significantly higher in the patients who experienced viral rebounds than in those who did not (Table 3, Supplementary Figs. 1-3).
Viral rebound was not related with the relapse of clinical symptoms. Ct values of E gene decreased at the time of viral rebound in 6 patients (40%), RdRp gene in 7 patients (47%), and N gene in 9 patients (60%) compared with the viral loads of the first RT-PCR (Fig. 2).

Changes in cycle threshold (Ct) values for each target gene from the first real-time reverse transcription polymerase chain reaction (RT-PCR) performed after admission (when the patients met the clinical criteria for release from quarantine) to the repeated RT-PCR at the first viral rebound. Red lines, increased viral loads; blue lines, decreased viral loads. E, envelope; RdRp, RNA-dependent RNA polymerase; N, nucleocapsid.
A significant number of patients (29%, 9 of 31) still had high or intermediate viral load when they met the clinical criteria of release from quarantine. The duration of viral detection was not different between symptomatic and asymptomatic patients. The duration of viral detection was significantly longer in the patients with viral rebounds than in those with no rebounds. (Table 3).
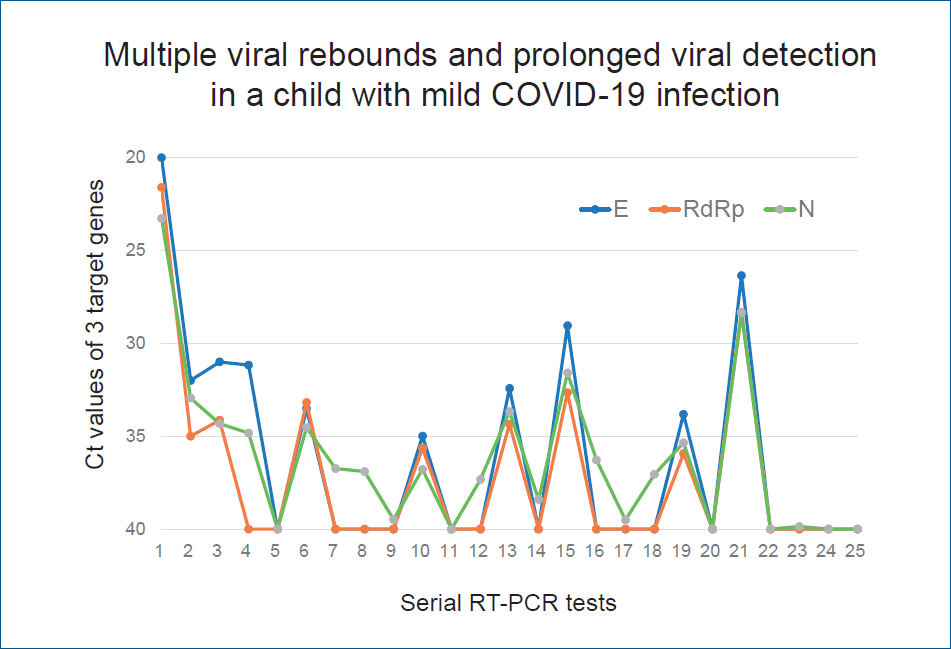
In this study, the mean interval between diagnosis and the first measurement of Ct values was 9 days (range, 5–13 days). Our patients received first negative results at a mean of 19.2 days (range, 7–57 days) after confirmed diagnosis. The mean time interval between diagnosis and 2 consecutive negative RT-PCR tests taken 24 hours apart, the mean duration of viral detection, was 25.6 days (range, 9–62 days). Twenty patients (65%) had viral detection within 4 weeks after diagnosis and 11 patients (35%) for more than 4 weeks (Table 3). Fig. 3 shows the chronological change of Ct values in the patients with prolonged viral detection for more than 4 weeks. It showed that dynamics of Ct values of 3 target genes in each patient are frequently discordant.
Discussion
In our study, we investigated the viral load in the pediatric patients admitted with COVID-19 who had mild disease or no symptoms. Our results showed that a significant number of patients still had high or intermediate viral load when they met the clinical criteria of release from quarantine. Nearly half of our patients experienced viral rebounds and prolonged viral detection during the course of COVID-19.
Many previous studies have reported that most children with COVID-19 are infected through contact with infected family members [12,13], which is consistent with our results. Our results showed that the proportion of transmission within family was highest when only mother was infected, which might be because mothers usually have close and prolonged contacts with their children. However, the source of transmission was not identified in 7 children (23%) who were tested because of mild URI symptoms. These results suggest that the children, even though they have no household contact and present only mild URI symptoms, need to be tested for COVID-19 during epidemics. However, considering low infection rate in pediatric population, this might cause unnecessary painful experiences in many children and a significant economic burden to be imposed on the national health care system.
Many previous studies have reported that children are less likely to be infected with SARS-CoV-2 compared with adults [4,5,7]. In Korea, the proportion of children under 19 years of age was 6.2% dated March 12, 2020 [14]. In this study, the children under age 3 accounted for the lowest proportion (6.5%) and the teenagers, aged 13–18 years, the highest proportion (38.7%). This high proportion in teenager group can be explained by the fact that the first outbreak in Daegu city was connected to certain religious organization.
In Korea, COVID-19 screening stations were set up to increase access to diagnostic testing during early phase of the outbreak. Contacts identified by epidemiological investigation were subject to self-quarantine for the maximum incubation period (14 days), which was strictly monitored by the government. Moreover, forced social distancing and postponement of the new semester of all kindergartens and schools in the whole country might have contributed to reduce the infection rate in children.
In the present study, the patients presented with only mild URI symptoms or were asymptomatic. They all made a full recovery, and none of them needed oxygen supplement or antiviral treatment. In addition to the pediatric patients with COVID-19 enrolled in our study, there were many others who were asymptomatic or had mild disease, and therefore, stayed at non-hospital isolation facilities called Living and Treatment Centers. Loss of smell or taste, reported to be frequently observed in the adult patients [15], was not seen in our patients. Previous studies have reported that many children with COVID-19 are asymptomatic or have milder symptoms than adults and generally have a good prognosis [4,6,13,16] and these findings are consistent with our results. A small proportion of children progressed to severe illness requiring intensive care and even death [4-6,17]. Early reports from China showed a relatively high proportion of severe cases among children younger than 1 year or those with underlying conditions [4,5]. In this study, no infant under age 1 was enrolled and all patients had mild disease or were asymptomatic, and so, we could not assess any risk factor for severe disease.
The previous studies have reported that SARS-CoV-2 viral loads were highest around a few days after symptom onset, and decreased over the following 1–3 weeks [18-20]. In the present study, unlike other studies, the Ct values of RT-PCR tests were evaluated when the patients met the clinical criteria for release from quarantine, and we found that 29% of the patients still had high or intermediate viral load when they had no more symptoms. Moreover, our study showed no difference in viral load among different age goups and also among 3 patient groups; asymptomatic, symptomatic, and presymptomatic group. Our findings seem to be consistent with the previous results that showed similar viral load irrespective of age and disease severity in children with COVID-19 [21,22]. On the other hand, a study in adult patients showed that viral load was correlated with disease severity [23], and another study in pediatric patients reported that young children under 5 years of age had significantly higher viral load compared with older children and adults [24]. Further investigation is needed to clarify the clinical significance of viral load in the children with COVID-19.
A recent study which enrolled 110 children with COVID-19 in China has reported that median period of viral detection in upper respiratory tract specimens was 15 days (range, 5–37 days), and symptomatic infection was associated with more prolonged duration of viral detection compared with asymptomatic infection [25]. In our study, mean time interval between diagnosis and 2 consecutive negative RT-PCR tests was 25.6 days, and there was no difference in the duration of viral detection among 3 groups of patients; asymptomatic, symptomatic, and presymptomatic groups. Difference in sample size, age distribution, and disease severity in symptomatic patients may explain the different results between the previous and the present study.
Nearly half of the patients enrolled in our study showed viral rebound after they received the first negative RT-PCR tests, and 8 of them had multiple rebounds until they showed 2 consecutive negative tests. Viral rebounds contributed to the prolonged viral detection, and duration of hospitalization was significantly longer in the patients with viral rebounds than in those without. Several studies have reported recurrent detection of SARS-CoV-2 in adult patients with COVID-19 [26-28], but not in pediatric patients yet. We expected high viral load in the patients who subsequently experienced viral rebounds, but the viral load of each target gene was significantly lower in the patients with viral rebounds than in those with no rebounds. In some patients, viral loads increased at the time of viral rebound, but viral rebound was not related with the relapse of clinical symptoms. A recent study reported that viral rebound was observed in 35.9% of the adult patients after the first negative RT-PCR results and the patients with viral rebounds had a lower viral load than those with no rebounds [28]. Our findings in the children with COVID-19 seem to be consistent with this previous study. Although it is not clear yet that frequent viral rebound and prolonged viral detection in respiratory specimens is a distinct feature of SARS-CoV-2, viral detection more than 2–3 weeks might be a natural course of COVID-19.
Most previous studies which reported the dynamics of viral load in children with COVID-19 included the pediatric patients who were treated with antiviral agent like interferon-α regardless of disease severity according to each nation’s guidelines [12,13,29-34]. Although the role of antiviral therapies in COVID-19 is not determined yet [35], as in SARS-CoV or other virus infection, interferon-α might affect the dynamics of viral load in the children with COIVD-19 [36]. In our study, all but 1 patient did not require any antiviral treatment, and we were able to show the natural course of viral load in the asymptomatic or mild pediatric cases. One patient was treated with IVIG for mild pneumonia with prolonged mild fever and he showed no viral rebound and the duration of viral detection was 17 days. Defining the natural history of the virus may indicate when the RT-PCR tests would be positive or negative during the illness. The children with COIVD-19 could be important transmitters in the community, because they usually had no symptoms or only mild disease. Our results seem to suggest the bases for designing strategies to control infection during COVID-19 outbreaks.
However, the relationship between vial load and infectiousness has not yet been clearly determined for COVID-19. A previous study reported that transmission could occur before symptom onset and infectiousness declined relatively quickly within 7 days of illness onset [37]. In our study, we could not measure the infectiousness in the patients who showed viral rebounds and prolonged viral detection which resulted in the prolonged hospitalization after clinical improvement. Many adolescent patients were anxious and depressed because of prolonged isolation. Further studies should be needed to clarify the relationship between vial detection and transmission during the course of COVID-19.
In the present study, to the best of our knowledge, we described for the first time that viral rebounds were frequently observed in the pediatric patients with COVID-19 until they showed 2 consecutive negative RT-PCR tests to be released from quarantine. We also analyzed first the 3 target genes of SARS-CoV-2 virus in the pediatric patients. However, our study had some limitations. The first one is the small numbers of the subjects enrolled in our study. Many more pediatric patients with COVID-19 who had mild disease or no symptoms were isolated at non-hospital isolation facilities with their families. Another limitation is that virus isolation in cell culture was not done and we could not determine the relationship between viral detection and infectivity.
In conclusion, our study showed that the pediatric patients with COVID-19 usually had mild disease or no symptoms. A significant number of the patients still had relatively high viral load when they had no more clinical symptoms. Nearly half of the patients experienced viral rebounds and showed prolonged viral detection. Our results suggest that further studies are needed to determine the relationship between viral load or viral rebound and infectiousness in COVID-19.
Supplementary materials
Supplementary Figures 1-3 can be found via https://doi.org/10.3345/cep.2020.02033.
Cycle threshold (Ct) values of envelope (E) gene in patients with and without viral rebounds.
Cycle threshold (Ct) values of RNA-dependent RNA polymerase (RdRp) gene in patients with and without viral rebounds.
Cycle threshold (Ct) values of nucleocapsid (N) gene in patients with and without viral rebounds.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.