Retinopathy of prematurity: a review of epidemiology and current treatment strategies
Article information
Abstract
Retinopathy of prematurity (ROP) is among the most common causes of childhood blindness. Three phases of ROP epidemics have been observed worldwide since ROP was first described in the 1940s. Despite advances in neonatal care, the occurrence of ROP and associated visual impairment has been increasing somewhere on Earth and remains difficult to control. Conventional treatment options for preventing ROP progression include retinal ablation using cryotherapy or laser therapy. With the emergence of anti-vascular endothelial growth factor (anti-VEGF) treatment for ocular diseases, the efficacy and safety of anti-VEGF therapy for ROP have recently been actively discussed. In the advanced stage of ROP with retinal detachment, surgical treatment including scleral buckling or vitrectomy is needed to maintain or induce retinal attachment. At this stage, the visual outcome is usually poor despite successful anatomical retinal attachment. Therefore, preventing ROP progression by timely screening examinations and treatment remains the most important part of ROP management.
Key message
There have been global tri-phasic epidemic periods of retinopathy of prematurity (ROP). In recent years, its incidence has reportedly been 10%–40% depending on country and study population. Current treatment strategies for ROP include laser photocoagulation, surgical treatment, and anti-vascular endothelial growth factor treatment, the role of which has drawn attention in recent years.
Introduction
Retinopathy of prematurity (ROP) is a leading cause of childhood vision loss worldwide [1]. Approximately 32,300 infants worldwide are diagnosed with irreversible vision impairment due to ROP annually, of which approximately 20,000 become blind or severely visually impaired [2]. Despite significant advances in neonatal care, the worldwide number of infants with ROP has been increasing as the survival rate of premature babies has increased. While much progress has been made in research into the pathophysiology and treatment of ROP over the past few decades, its occurrence and the resulting blindness remain problematic.
To prevent the acquired childhood blindness caused by ROP, it is important to understand its epidemiology and develop appropriate treatment plans. This review addresses recent epidemiology and treatment strategies for ROP.
Epidemiology
Since it was first described in 1942 [3,4], ROP has become recognized as the primary cause of childhood blindness. Historically, there have been global tri-phasic epidemic periods of ROP and ROP-induced blindness [5].
1. Three phases of ROP “epidemics”
The first epidemic was observed in the late 1940s and early 1950s, when ROP occurred due to unrestricted oxygen use without adequate monitoring [3,4]. During this period, the mean birth weights (BWs) of ROP babies were 1,370 g (range, 934–1,843 g) and 1,354 g (range, 770–3,421 g) in the United Kingdom (UK) and United States (US), respectively [6,7]. The second epidemic started in the late 1960s and early 1970s when the survival of smaller, less mature infants increased with numerous advances in neonatal care in industrialized countries with well-developed neonatal units [8,9]. Advances in technology to control the environmental conditions of premature infants have improved the survival of extremely premature infants. In the early 1990s, it became apparent that an epidemic of ROP blindness was emerging in middle-income countries with developing neonatal intensive care, referred to as the third epidemic [7,10].
There have been several explanations for this third epidemic. In middle-income countries, even if there are sufficient resources to save premature infants, either neonatal care levels are inadequate to prevent ROP or there are insufficient resources to examine and treat at-risk babies [7,11]. Advanced training for neonatologists, ophthalmologists, and neonatal nurses is often lacking, oxygen saturation is not monitored, and ROP is seen in the smallest infants if they do survive as well as in more mature preterm infants [12]. These regions include middle-income regions of Latin America, East and South Asia, Eastern Europe, and Central Asia according to Blencowe et al. [2] During this third epidemic, the mean BWs of ROP babies needing treatment ranged from 737 to 763 g (range, 440–1,785 g) in the UK, Canada, and the US, while mean gestational age (GA) ranged from 25.3 to 25.6 weeks (range, 22–32 weeks) [12]. Meanwhile, babies affected in low- and middle-income countries have a far wider range of BWs and GAs [13-15]. These differences occur among regions, and sometimes even among facilities in the same region with differing resource and care levels [16].
2. Recent population-based ROP epidemiological studies
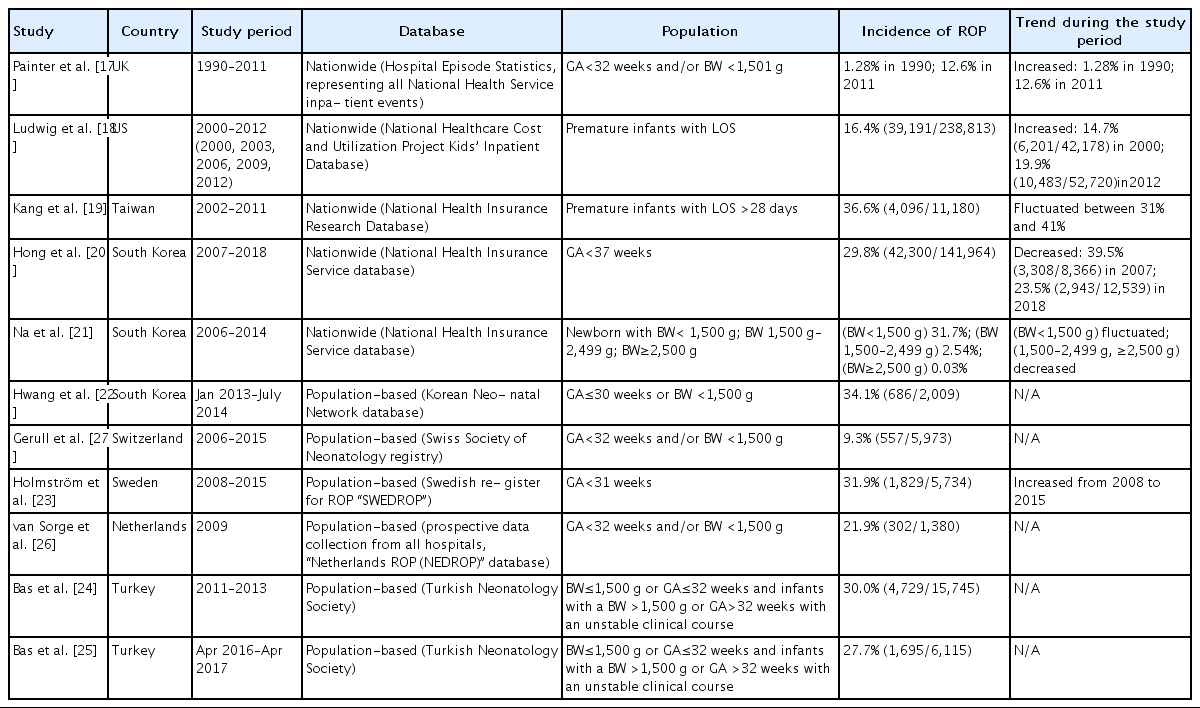
Several nationwide or population-based studies have used various definitions of population. Table 1 summarizes studies published in the last 10 years. The trend in the incidence of ROP varied among countries, study periods, and study populations. In a nationwide study in the UK, the incidence of ROP in 2011 was 12.6% among infants with a GA<32 weeks and/or BW<1,501 g [17]. In the US, between 2000 and 2012, it was reportedly 16.4% among premature infants with a length of stay (LOS) in the hospital longer than 28 days [18]. In Taiwan, between 2002 and 2011, a 36.6% incidence of ROP was reported among premature infants using the same definition [19]. In South Korea, there were 2 nationwide studies: one reported an incidence of 29.8% among infants with a GA<37 weeks between 2007 and 2018 [20], while the other reported an incidence of 31.7% among premature infants with a BW<1,500 g between 2006 and 2014 [21].
In several countries, there have been population-based studies using national registry database of national neonatology societies or national ROP consortia [22-27]. The database is based on the hospital records of neonatal intensive care units (NICUs), and these studies have roughly the following common composition of the study population: very low birth weight infants (VLBWIs, BW<1,500 g), those with a GA<30 to 32 weeks, or premature infants with an unstable clinical course. Reports from South Korea, Sweden, and Turkey showed that the incidence of ROP was around 30% (27.7%–34.1%) [22-25], while the Netherlands and Switzerland reported an incidence of 21.9% and 9.3%, respectively [26,27]. In South Korea, there were annual reports between 2014 and 2018 from the Korean Neonatal Network (KNN), a national multicenter neonatal network based on a prospective web-based registry for VLBWIs supported by the Korea Centers for Disease Control and Prevention [28]. The KNN data included approximately 70% of the overall admissions of VLBWIs born in the nation [28]. According to their reports, ROP occurred in 32.2% (3,039 of 9,435) of VLBWIs born between 2014 and 2018 [29]. The reported incidence according to GA and ROP stage is presented in Supplementary Table 1.
A detailed incidence according to ROP stage or need for treatment was also provided in these population-based studies. The incidence of premature infants requiring treatment was 0.2% in 1990 and 1.5% in 2011 in infants with a GA<32 weeks and/or BW<1,501 g in the UK (cryotherapy or laser coagulation) [17], 1.5% (2,284 of 153,706) among premature infants with an LOS>28 days in 2006, 2009, and 2012 in the US (laser coagulation or vitreoretinal surgery) [18], and 2.1% (238 of 11,180) among premature infants with an LOS>28 days between 2002 and 2011 in Taiwan (cryotherapy, laser coagulation, intravitreal anti-vascular endothelial growth factor [anti-VEGF] or vitreoretinal surgery) [19], and 0.9% (1,247 of 141,964) among premature infants with GA<37 weeks between 2007 and 2018 in South Korea (cryotherapy, laser coagulation, or vitreoretinal surgery) [20].
In a Swedish study, the incidence of ROP was 9.5% (544 of 5,734) for stage 1, 11.6% (666 of 5,734) for stage 2, 10.4% (597 of 5,734) for stage 3, 0.2% (11 of 5,734) for stage 4, and 0.2% (11 of 5,734) for stage 5 among infants with a GA<31 weeks between 2007 and 2015 [23]. A total of 5.7% (329 of 5,735) required treatment for ROP. According to a study from the Swiss Society of Neonatology, the incidence of ROP by stage was 4.6% (275 of 5,973) for stage 1, 2.9% (173 of 5,973) for stage 2, 1.8% (105 of 5,973) for stage 3, 0% (1 of 5,973) for stage 4, and 0.1% (3 of 5,973) for stage 5 among infants with a GA<32 weeks between 2006 and 2015 [27]. In this report, the incidence of ROP requiring treatment (cryotherapy, laser coagulation, or intravitreal anti-VEGF) was 1.2% (76 of 5,973). In a Dutch study, the incidence of infants with ≥stage 3 ROP was 2.1% (29 of 1,380) and those with stage 1 and 2 ROP was 19.8% (273 of 1,380) among premature infants with a BW≤1,500 g and/or GA≤32 weeks in 2009 [26]. In an earlier Turkish study conducted between 2011 and 2013, the incidence of infants with ≥stage 3 ROP was 5.0% (790 of 15,745), that of those requiring treatment (laser coagulation or vitreoretinal surgery) was 5.1% (810 of 15,745) among infants with BW≤1,500 g, GA≤32 weeks, or with an unstable clinical course [24]. The later Turkish study conducted in 2016–2017 reported that the incidence of infants requiring treatment (laser coagulation, intravitreal antiVEGF, or vitreoretinal surgery) was 6.8% (414 of 6,115) [25].
In addition to these population-based studies, many multitertiary center-based studies have been published. In 2 NICUs in Hong Kong, among neonates with a BW≤1,500 g and/or GA≤32 weeks who were screened for ROP between January 2007 and December 2012, 18.5% (95 of 513) tested positive [30]. Several studies have been conducted in regions of China. A multicenter study conducted in Shanghai in 2012–2016 showed an ROP incidence of 15.9% (892 of 5,606) among all infants undergoing ROP screening [31]. In Southwest China, 12.8% (206 of 1,614) of premature infants with a GA<37 weeks and BW≤2,500 g were diagnosed with ROP between 2009 and 2012, which showed a decreasing trend from 17.1% in 2009 to 11.0% in 2011 [32].
Pathophysiology
The development and progression of ROP are characterized by abnormal neovascularization, which typically occurs in 2 postnatal phases [33]. In the first phase, immediately after birth up to 32 weeks’ postmenstrual age, normal vascular growth in the retina stops due to hyperoxia, which is referred to as “oxygen toxicity.” [34] In premature infants, even room air leads to a hyperoxic environment compared to the intrauterine environment [35]; moreover, oxygen supplement in cases with respiratory distress worsens this hyperoxia. Hyperoxia causes both cessation of retinal vessel growth and partial regression of existing vessels in this phase [36]. The second phase follows with hypoxia-induced pathological vasoproliferation [34]. Incomplete vascularization causes the retina to become hypoxic, leading to the release of various angiogenic factors including VEGF and erythropoietin and subsequently to neovascularization, leading to intraocular fibrosis and retinal detachment [33,34].
Classification
1. Zones and stages
ROP is categorized according to the International Classification of Retinopathy of Prematurity (ICROP), which was first published in 1984 [37] and revised in 2005 [38]. ROP is classified according to the 3 “zones” of the retina, which indicates the location of the leading edge of retinal vascularization, and the severity of the disease (“stage”) in these zones [38]. The zones of the retina are shown in Fig. 1A. Zone 1 is a circular area centered on the optic disc, the radius of which is twice the distance between the optic disc and the macula. Zone 2 is an extended circular area centered on the optic disc to the nasal ora serrata, while zone 3 includes the remaining crescent area of the temporal retina. ROP in zone 1 is most likely to become aggressive and severe, while ROP in zone 3 is rarely aggressive [39]. Stage is defined according to funduscopic findings (Fig. 1B). In stage 1, there is a demarcation line between the normally vascularized retina and the peripheral avascular retina. In stage 2, the demarcation line becomes an elevated ridge. There are no pathologic new vessels in stages 1 and 2, and they are more likely to regress spontaneously in these stages. In stage 3, extraretinal neovascularization has the potential to cause traction on the retina, which can progress to partial or total retinal detachment (stages 4 and 5, respectively). Fig. 2 shows retinal fundus images of stage 1–4 ROP. The visual prognosis is very poor in these stages [38]. In addition to zone and stage categorization, the presence of increased venous dilation and arteriolar tortuosity of posterior pole vessels (so-called “plus disease”) is an ominous sign of progressive disease that indicates vascular shunting and severe ROP. In the revised ICROP classification, “pre-plus disease” was defined as an active ROP state in which the vascular changes are more prominent than normal, but the features are insufficient to be diagnosed as plus disease. This referred to a prestage that could develop into plus disease over time [38,40].

Classification of retinopathy of prematurity (ROP) according to zone and stage. (A) Scheme of retina of the right eye representing 3 distinct zones. (B) ROP severity is classified as stages. In stage 1, a demarcation line is observed; in stage 2, an elevated ridge is observed; in stage 3, an extraretinal fibrovascular proliferation with neovascularization is observed, which can lead to partial (stage 4) or total retinal detachment (stage 5).
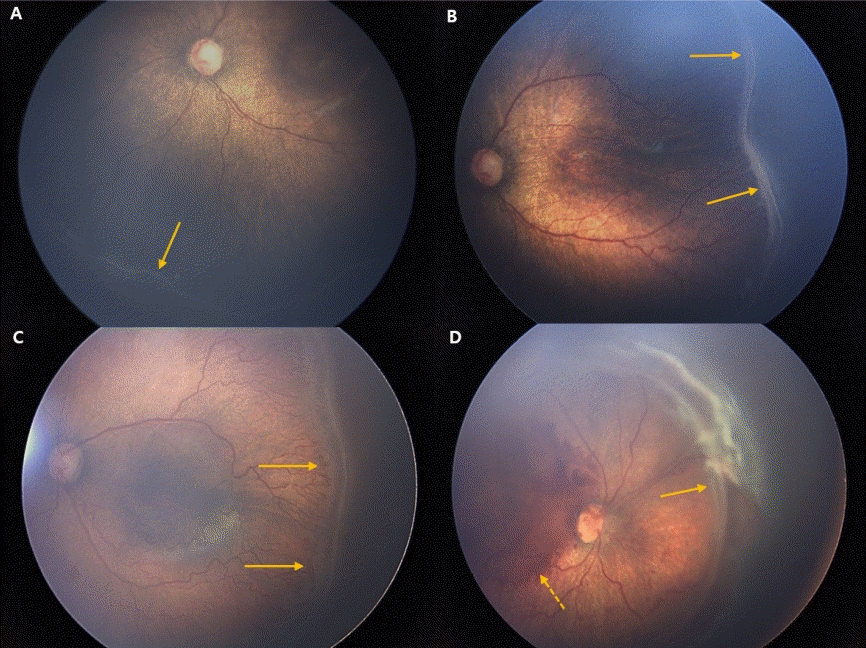
Fundus photos of stages 1–4 retinopathy of prematurity (ROP). (A) In stage 1, a demarcation line (arrow) between a normally vascularized retina and the peripheral avascular retina is shown. (B) In stage 2, the demarcation line becomes an elevated ridge (arrow). (C) In stage 3, extraretinal fibrovascular proliferation appears (arrow). (D) Partial retinal detachment (arrow) in the nasal side of the fundus and preretinal hemorrhage (dashed arrow) are shown. (Fundus photos courtesy of Dr. Sang Jin Kim).
2. Aggressive posterior ROP
In addition to this staged ROP, aggressive posterior ROP (AP-ROP) indicates a more virulent form of ROP in extremely low BW babies and involves very central neovascularization with plus disease. AP-ROP is limited to the posterior pole of zone 1 or 2 and does not classically progress through stages 1–3 of ROP, and it can progress very quickly to retinal detachment [39].
3. Types
The zone and stage of ROP can be combined and reclassified into 2 types according to the study of the Early Treatment for Retinopathy of Prematurity (ETROP) [41]. Type 1 indicates eyes with significant changes that require treatment, while type 2 indicates changes that do not require treatment at that moment but must be carefully followed up [42]. The stages of ROP and other current terminology are summarized in Table 2. Type 1 now includes AP-ROP [38].
4. Threshold and prethereshold
The terms threshold and prethreshold were originally introduced in the Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) study in the late 1980s to determine when to begin treatment [40,43]. Threshold ROP was defined as a condition with a 50% risk of retinal detachment if left untreated, which included ROP of at least 5 contiguous or 8 cumulative clock hours of stage 3 ROP in the presence of plus disease in zones 1 or 2. In the presence of threshold disease, treatment was recommended [43]. Prethreshold ROP was defined as: zone 1, any ROP; zone 2, stage 2 ROP with plus disease; zone 2, any amount of stage 3 ROP and no plus disease; or zone 2, stage 3 ROP with plus disease but less than the required threshold clock hours. Prethreshold ROP was advised to be followed up. This prethreshold ROP was divided into 2 types in the revised categorization according to the ETROP trial in 2003: type 1 high-risk prethreshold ROP, defined as zone 1 plus with any stage, zone 1 stage 3 with no plus, and zone 2 stage 2 or 3 plus; and type 2 low-risk prethreshold ROP, defined as zone 1 stage 1 or 2 without plus disease and zone 2 stage 3 without plus disease [41]. Threshold ROP and type 1 high-risk prethreshold ROP were incorporated in type 1 ROP requiring immediate treatment, and follow-up is recommended for type 2 low-risk prethreshold ROP.
5. Referral warranted ROP
A more recent classification was suggested by the Telemedicine Approaches for the Evaluation of Acute-Phase Retinopathy of Prematurity (e-ROP) study in 2014 [44]. The e-ROP study introduced referral warranted ROP (RW-ROP), defined as any ROP in zone 1 ROP, stage 3 ROP or worse, or plus disease, to identify those who needed evaluation by an ophthalmologist to consider treatment. RW-ROP in the e-ROP study was consistent with ROP that had at least a prethreshold severity in the CRYOROP and ETROP studies, while plus disease alone involved greater severity in the e-ROP study [45].
Current treatment strategies
Current indications for treatment are based on the ETROP study of type 1 ROP, which is characterized as zone 1, any stage ROP with plus disease; zone 1, stage 3 ROP without plus disease; zone 2, stage 2 or 3 ROP with plus disease [41]. Treatment should be initiated for type 1 ROP within 72 hours of its detection, ideally to minimize the risk of retinal detachment [46].
1. Cryotherapy
Conventional treatment focused on inhibiting aberrant intravitreal angiogenesis to prevent fibrovascular retinal detachment. Ablation of the peripheral avascular retina is believed to reduce the hypoxic retina that expresses angiogenic factors or treat cells expressing angiogenic factors. Cryotherapy was established in the late 1980s as a conventional treatment for ROP to ablate the avascular retina according to the CRYO-ROP study [43]. The CRYO-ROP study reported that cryotherapy for threshold ROP significantly improved anatomical outcomes and visual development versus no treatment [43,47] and that the structural and functional benefits of cryotherapy were maintained over the 15 years of follow-up [48]. These studies showed for the first time that there is an effective treatment for active ROP. At that time, the indication for treatment was threshold ROP, as described in section 3.4 [49]. Afterward, the indirect laser delivery system to the eye became widely available, and ETROP study series reported that treatment with laser photocoagulation or cryotherapy in type 1 ROP significantly improved outcomes compared to standard care of threshold ROP [41], which established the need for earlier treatment for type 1 ROP instead of waiting for threshold ROP to be reached.
However, cryotherapy reportedly causes more inflammation, which is involved in the pathogenesis of ROP [50], and showed poorer outcomes than laser treatment [51,52]. Some studies suggested that the development of myopia is more severe in cryotherapy-treated eyes than in laser-treated eyes [53], which seemed mainly due to increased lens thickness rather than axial length elongation [54]. Furthermore, lasers have practical advantages over cryotherapy with reduced requirements for general anesthesia and improved equipment mobility (if a portable diode laser unit is used) as well as the ability to more effectively treat the posterior retina (zone 1 ROP) [55].
As a result, cryotherapy has been used less commonly for severe ROP since the advent of indirect laser delivery systems in the late 1980s, and the American Academy of Ophthalmology recommends that laser photocoagulation be performed whenever possible for infants with ROP who meet the treatment criteria [55].
2. Laser photocoagulation
The current standard treatment option for severe ROP is laser ablation of the peripheral avascular retina. Since the ETROP trial study showed a reduction in unfavorable structural outcomes after earlier treatment for high-risk prethreshold ROP [41], laser treatment is considered the standard for reducing vascular activity and consequently alleviating the need for surgery for fibrovascular tractional retinal detachment [56]. Laser treatment is currently considered immediately (within 72 hours in the US [56]) for type 1 ROP, while careful monitoring (“wait and watch”) is recommended in type 2 ROP [57].
The procedure can be performed in the operating room or in the neonatal care unit under general anesthesia or sedation [58]. As it is difficult to distinguish between flat neovascularization overlying the avascular retina and normal retinal vasculature and direct treatment increases the risk of vitreous hemorrhage [56], 2-stage laser treatment reportedly safely causes regression with a lower risk of vitreous hemorrhage in eyes with flat neovascularization [59]. The first stage involves applying the laser to the avascular retina up to the flat neovascularization; then, once regression occurs, the second stage involves adding a laser to the newly created avascular bed at the point of flat neovascularization to prevent subsequent neovascularization [59].
Nevertheless, the limitations or side effects of ablation of the avascular retina remain. While evidence of retinal vessels growing between treated areas toward the ora serrata is lacking, there is strong evidence that eyes underwent spontaneous regression of preretinal neovascularization and vascularization of the previously peripheral avascular retina [60,61]. Disappointing outcomes have also been observed in some laser-treated eyes, especially in cases of zone 1 ROP or AP-ROP or cases of insufficiently treated ROP [43,62]. Laser-induced visual field constriction, which is less than that predicted on a fundus examination from laser treatment in zone 2, exists in zone 1 ROP, although this is difficult to assess due to the frequent poor visual outcomes of such children [63]. Eyes with AP-ROP and flat neovascularization are currently considered for treatment with anti-VEGF agents [46].
3. Anti-VEGF therapy
The introduction of intravitreal anti-VEGF therapy is a recent development in the treatment of ROP [42,64]. Experimental evidence suggests that regulation of the VEGF signaling pathway could both inhibit preretinal neovascularization [65,66] and facilitate developmental intraretinal angiogenesis, thereby reducing the incidence of hypoxic peripheral avascular retina when the infant is removed from high supplemental oxygen [67,68]. The role of anti-VEGF therapy in ROP has drawn attention in recent years due to the need for less destructive treatment. Retinal ablation, including laser photocoagulation and cryotherapy, is designed to destroy the peripheral avascular retina, while intravitreal anti-VEGF injection promotes the restoration of VEGF signaling to physiological levels locally in the retina, which seems an ideal treatment. In addition, it is easier and quicker to administer since the procedure can be performed at the bedside under local anesthesia, requires less specialized equipment, and may be used in infants with corneal or lens opacity or poor pupillary dilation in whom laser photocoagulation is impossible to perform [64]. Based on these theoretical advantages and attractions, clinical trials have used various anti-VEGF agents, including bevacizumab, ranibizumab, and pegaptanib.
There have been a few multicenter trials of anti-VEGF therapy for ROP. The first clinical trial, Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity (BEAT-ROP) in 2011, reported significant treatment effects of bevacizumab over laser therapy for zone 1 (but not zone 2) disease in infants with stage 3+ ROP [64,69]. The 5-year outcomes of the BEAT-ROP study showed a recurrence rate of 7.2% after intravitreal bevacizumab therapy (0.625 mg) with the risk factors of AP-ROP, a prolonged hospital stay, and a lower BW [70].
In 2018, a Cochrane review of intravitreal anti-VEGF treatment for ROP reported that intravitreal bevacizumab or ranibizumab as a monotherapy reduced the risk of refractive errors but did not reduce the risk of retinal detachment or ROP recurrence in type 1 ROP [71]. Additionally, this intervention might reduce the risk of ROP reoccurrence in zone 1 ROP cases, which could potentially increase the risk of recurrence requiring treatment in zone 2 ROP cases. This review also concluded that further research is needed to assess the impact of anti-VEGF agents on structural and functional outcomes and delayed systemic effects, including neurodevelopmental outcomes in childhood.
The Pediatric Eye Disease Investigator Group (PEDIG) ROP phase 1 study investigated the efficacy of lower-dose bevacizumab and found that, even at a dose of 0.031 mg, it was effective in a small sample of infants with type 1 ROP through 6 months [72]. However, the number of infants was too small to make a definite conclusion about the optimal dosage. Future studies will compare a selective dosage to laser with outcomes of efficacy of treatment, extension of vascularization of the previously avascular retina, ROP reactivation, and neurodevelopmental outcomes.
The Comparing Alternative Ranibizumab Dosages for Safety and Efficacy in Retinopathy of Prematurity (CARE-ROP) study, an interventional investigator-initiated study, was performed in Germany and investigated the use of ranibizumab 0.12 mg and 0.20 mg in infants with ROP, corresponding to 24% and 40% of the adult dose, respectively [73]. The CARE-ROP study had the primary endpoint (the proportion of infants who did not require rescue therapy) at 24 weeks and 5 years of follow-up. The results suggested that both doses were equally successful at controlling ROP, and blood VEGF levels were not altered in either group, indicating limited systemic drug exposure. Recently reported 1-year follow-up ophthalmic outcomes of the CARE-ROP study reported that anti-VEGF treatment with ranibizumab appears safe and effective; however, late reactivations must be taken very seriously and follow-up examinations require the utmost care [74].
The RAnibizumab compared with laser therapy for the treatment of INfants BOrn prematurely With retinopathy of prematurity (RAINBOW) study used ranibizumab (0.2 mg, 0.1 mg), which is cleared more rapidly from the blood and eyes compared to laser treatment [75]. The RAINBOW study was the first global phase 3 randomized controlled trial of ranibizumab for ROP. A total of 225 patients from 87 centers were enrolled and randomized 1:1:1 to ranibizumab 0.2 mg, ranibizumab 0.1 mg, or laser treatment. The study did not achieve statistical significance, but the authors concluded that the ranibizumab 0.2 mg dose could be superior to laser treatment. This trial was conducted on a more severe level of treatment-warranted ROP than type 1 ROP tested in the ETROP and ROP1 studies, mainly including eyes with zone 2 ROP. Recurrence at 6 months occurred in 31% of patients, but no reduced blood VEGF level was noted from either dose of ranibizumab at 1 month [75]. The 5-year RAINBOW extension study is currently ongoing, with results expected in 2022. Based on the RAINBOW results, ranibizumab was approved in the European Union in September 2019 for the treatment of ROP in preterm infants with zone 1 (stage 1+, 2+, 3, or 3+), zone 2 (stage 3+), or AP-ROP disease.
Regarding long-term ophthalmic outcomes, intravitreal bevacizumab injection reportedly induces less myopia than laser treatment [76,77].
Despite the promising outcomes of anti-VEGF treatment in ROP, there are concerns about anti-VEGF therapy, including reports that the effect may be transient with later ROP recurrence [78-81], a lack of knowledge about its effect on normal angiogenesis in the organs of the developing preterm infant, and potential adverse effects on the neural retina. Furthermore, several studies have reported that anti-VEGF agents enter the systemic circulation following injections into the eye [82,83]. Therefore, there is a risk that anti-VEGF agents injected into the eyes of infants can impede the development of other central nervous system structures or organs. Long-term follow-up studies with adequate numbers of patients are needed to establish safety.
For these reasons, the American Academy of Pediatrics suggests that detailed informed consent should be obtained if anti-VEGF therapy is contemplated [46]. It recommends that ROP eyes treated with anti-VEGF therapy should be monitored until the postmenstrual age of at least 65 weeks and that caution and clinical judgment are required to determine when surveillance can be safely terminated in individual cases. Infants treated with anti-VEGF medications require particularly close follow-up during the time of highest risk for disease reactivation (postmenstrual age 45–55 weeks) [46]. The follow-up of treated infants should be recommended by the treating ophthalmologist.
4. Surgical treatment
Once retinal detachment occurs, surgical treatment is needed; regardless, the visual outcomes are generally poor [84,85]. Surgical options include scleral buckling or lens-sparing vitrectomy. The goals of surgery are to release vitreoretinal tractional components extending between the ridge and the anterior eye, the peripheral retina extending to the ora serrata, the optic disc, and the ridge creating “circumferential” traction.
Scleral buckling has been proposed for stage 4 ROP to reduce traction and stabilize vascular activity [86]. In eyes with peripheral detachments, in which it is difficult to handle tractional forces without removing the lens, scleral buckling is still advocated [87]. Scleral buckling is also considered for eyes with rhegmatogenous retinal detachment due to peripheral breaks, which may occur adjacent to the previous laser spots or may be caused by traction on the thin retina [56]. However, there are risks of concern, such as a risk of perforation during the procedure as the infant sclera is thinner than the adult sclera, and an increased risk of anisometropic amblyopia by inducing myopic change. After scleral buckling is performed, a second surgery may be required to divide the buckle as the eye grows, often at 3–6 months of age [56].
Lens-sparing vitrectomy has been ideally performed for stage 4 ROP in posterior zone 2 or zone 1, or stage 5 ROP [88,89]. Several studies have shown that for zone 2, stage 4 ROP following laser treatment, outcomes are better with lens-sparing vitrectomy than primary scleral buckling [90,91].
Previous treatments have focused on retinal reattachment in stage 5 ROP, and while successful anatomical reattachment was technically possible, visual outcomes were often limited [92]. Later, the treatment strategy was changed to prevent stage 5 ROP with lens-sparing vitrectomy in cases of progressive stage 4 ROP. ROP progression may induce recurrent vascular activity with retinal detachment, which may reduce surgical success [93]. Several features are associated with progressive stage 4 ROP, including 2 or more of the following: 6 or more clock hours of ridge elevation, recurrent or persistent plus disease in 2 quadrants, and vitreous condensation or haze [94]. Also important is the presence of vitreous hemorrhage [95] or vitreous organization [96]. It is helpful to determine the angle between the posterior retinal veins, with increasingly acute angles being suggestive of peripheral traction. Vascularly active ROP is treated to reduce activity [97] before surgery to improve outcomes [93].
In stage 5 ROP, retinal reattachment is performed in infants through 2 years of age. Even after successful reattachment, complications related to earlier ROP, including rhegmatogenous retinal detachment and cataracts requiring surgery to restore or preserve vision, can occur throughout life [98].
Prognosis
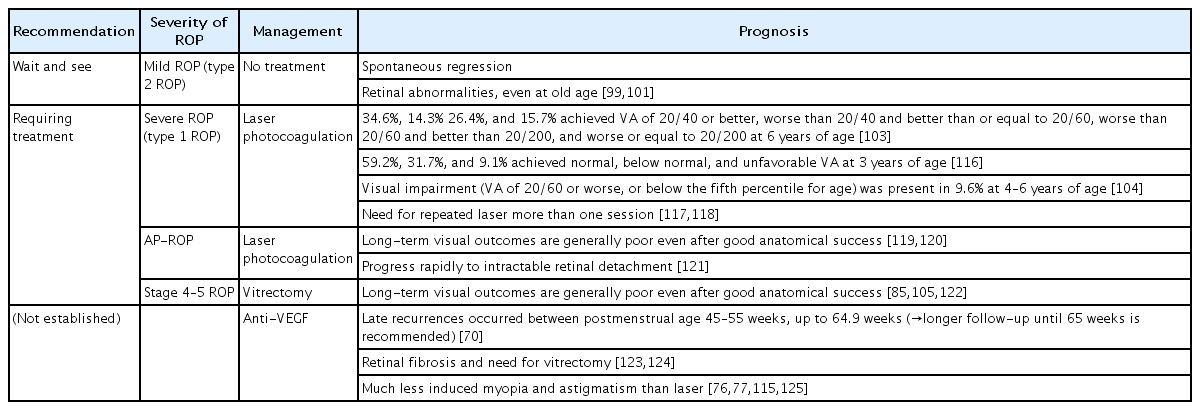
Table 3 shows a brief summary of prognosis after ETROP guidelines were adopted and intravitreal anti-VEGF therapy was introduced.
1. Eyes with ROP not requiring treatment
Most cases with mild ROP including stage 1 and 2 ROP and all ROP cases not meeting type 1 criteria usually resolve spontaneously after some time, and the visual prognosis is known to be associated with ROP severity in the acute phase [99]. Mild ROP may also affect visual prognosis, but results from previous epidemiologic studies differ [100]. While the impact of mild ROP on visual function is unclear, preterm birth itself may contribute to the risk of impaired visual function. Additionally, there is a lack of long-term visual acuity data in mild ROP that do not meet type 1 criteria, as the ETROP treatment guidelines were adopted in 2004. A recent multicenter-based study reported that 45% of those with ROP who did not require treatment showed visual acuity better than 20/40 at an average age of 34.5 years; however, 22% of them showed visual acuity of counting fingers or worse [99]. However, it should be noted that about 50% of the participants in this study were born even before the CRYO-ROP study. Late retinal abnormalities, including peripheral lattice degeneration, retinal tears, atrophic holes, or retinal detachment, have also been reported even later in life [99,101,102], which can affect visual prognosis if not treated in a timely manner.
2. Eyes with ROP requiring treatment
Among eyes with severe ROP requiring treatment, a high proportion of those untreated and a proportion of those treated develop structural changes including retinal scarring, distortion, or detachment with irreversible vision loss [100]. According to the ETROP study, 35%, 49%, and 75% of treated eyes with type 1 ROP achieved a visual acuity of 20/40 or better, 20/60 or better, and 20/200 or better at 6 years of age [103]. In a long-term follow-up analysis, visual impairment (visual acuity of 20/60 or worse or below the fifth percentile for age) was present in 9.6% of infants with stage 3–4 ROP or laser-treated ROP at 4–6 years of age [104]. Some eyes with stage 4 disease have limited vision following surgery, and the results for stage 5 are very poor even despite treatment [85,105,106].
3. Ophthalmic complications after ROP regression
ROP can lead to various short- and long-term ophthalmic complications, even after spontaneous regression or treatment. Such complications include early or late retinal detachment, cataracts, glaucoma, strabismus, refractive problems, amblyopia, and nystagmus [48,57,100]. According to the ETROP study, which included ROP infants with BW≤1,251 g born between October 1, 2000 and September 30, 2002 in multiple centers in the US, the prevalence of cataract at 6 months of age was 1.9% [107], while those of glaucoma, retinal detachment, and strabismus at 6 years of age were 1.67% [108], 16.2% [105], and 42.2% [109] and the prevalence of nystagmus among bilateral high-risk prethreshold ROP cases was 22% [57]. Varying degrees of refractive errors including moderate to high myopia, astigmatism, or hypermetropia after ROP have been reported at the age of 3.5–12 years [110-114]. According to a systematic review, mild ROP does not contribute to refractive errors, which is attributable to preterm birth; meanwhile, there is an increase in all refractive errors, frequently high myopia, following severe ROP [100]. Myopia is less severe following intravitreal anti-VEGF treatment versus laser photocoagulation or cryotherapy [22,115].
Conclusion
In the past few decades, neonatal care has developed significantly. Nevertheless, ROP is still the leading cause of childhood visual impairment worldwide. Understanding the heterogeneity of ROP epidemics worldwide is essential to reduce this considerable global burden. The most important aspect in ROP prognosis is preventing it before it progresses to an advanced stage, and in an effort to make it efficient, the classification system of ROP has been created and revised. Established or developing treatment options to date include cryotherapy, laser ablation, surgical treatment, and anti-VEGF therapy. Anti-VEGF agents, with the exception of one (ranibizumab) approved in Europe, are still used as off-label, which requires the establishment of its safety and effectiveness and will require long-term research results like other treatments.
Supplementary materials
Supplementary Table 1 can be found via https://doi.org/10.3345/cep.2021.00773.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We are thankful to Dr. Sang Jin Kim (Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea) for allowing us to use the fundus photographs.