Genetic factors in precocious puberty
Article information
Abstract
Pubertal onset is known to result from reactivation of the hypothalamic-pituitary-gonadal (HPG) axis, which is controlled by complex interactions of genetic and nongenetic factors. Most cases of precocious puberty (PP) are diagnosed as central PP (CPP), defined as premature activation of the HPG axis. The cause of CPP in most girls is not identifiable and, thus, referred to as idiopathic CPP (ICPP), whereas boys are more likely to have an organic lesion in the brain. ICPP has a genetic background, as supported by studies showing that maternal age at menarche is associated with pubertal timing in their offspring. A gain of expression in the kisspeptin gene (KISS1), gain-of-function mutation in the kisspeptin receptor gene (KISS1R), loss-of-function mutation in makorin ring finger protein 3 (MKRN3), and loss-of-function mutations in the delta-like homolog 1 gene (DLK1) have been associated with ICPP. Other genes, such as gamma-aminobutyric acid receptor subunit alpha-1 (GABRA1), lin-28 homolog B (LIN28B), neuropeptide Y (NPYR), tachykinin 3 (TAC3), and tachykinin receptor 3 (TACR3), have been implicated in the progression of ICPP, although their relationships require elucidation. Environmental and socioeconomic factors may also be correlated with ICPP. In the progression of CPP, epigenetic factors such as DNA methylation, histone posttranslational modifications, and noncoding ribonucleic acids may mediate the relationship between genetic and environmental factors. CPP is correlated with short- and long-term adverse health outcomes, which forms the rationale for research focusing on understanding its genetic and nongenetic factors.
Key message
· Mutations in the kisspeptin (KISS1), kisspeptin receptor (KISS1R), makorin ring finger protein 3 (MKRN3), and delta-like homolog 1 (DLK1) genes are associated with idiopathic central precocious puberty (ICPP).
· A few genes related to pubertal onset have been implicated in ICPP.
· Epigenetic factors such as DNA methylation, histone posttranslational modifications, and noncoding ribonucleic acids may be related to ICPP.
Graphical abstract
Introduction
Puberty is the transitional period from childhood to adulthood during which an individual matures gradually, develops secondary sexual characteristics, and becomes physically fertile. From a psychosocial perspective, a person develops cognitive function, self-reflection, and moral values during puberty [1]. Because the mechanisms of pubertal development have not been fully elucidated, it remains a great mystery of science. During the fetal and neonatal periods, the hypothalamic-pituitary-gonadal (HPG) axis is active; however, in the period before pubertal onset, this activity is repressed and the HPG axis becomes quiescent [2]. The axis is then reactivated during pubertal onset, which leads to pulsatile gonadotropin-releasing hormone (GnRH) secretion. GnRH is regulated by activators such as kisspeptin as well as inhibitors of the HPG axis [2]. In brief, GnRH secretion is results from increases in activators, including kisspeptin produced by kisspeptin, neurokinin B (NKB), and dynorphin (KNDy) neurons [3]. NKB and its receptor (neurokinin receptor 3, NK3R) play a role in the upstream regulation of kisspeptin.
The kisspeptin signaling pathway through kisspeptin receptors (KISS1R) on GnRH neurons controls pulsatile GnRH release. GnRH secretion leads to increases in luteinizing hormone and follicle-stimulating hormone from the pituitary, which results in the activation of sex steroid production (testosterone in males, estrogen and progesterone in females) and the production of sperm and mature oocytes. Activation of the HPG axis results in the progression of puberty, including breast development, pubic hair growth, and menstruation in girls and testicular enlargement, pubic hair growth, an increase in penile length, and voice changes in boys [4]. The average age at pubertal onset is 10–11 years in girls and 11–12 years in boys [4].
Precocious puberty
1. Definition
Based on this normal pubertal timing, precocious puberty (PP) has been defined as breast development before the age of 8 years in girls and testicular enlargement ≥4 mL before the age of 9 years in boys [5]. Concern has been raised regarding modification of the definition of PP because of the downward secular trend of pubertal onset. A Korean study showed a downward secular trend in menarche. The estimated mean age at menarche in girls born between 1986 and 1995 was 13.10 years, whereas that in girls born between 1980 and 1985 was 13.79 years [6]. The mean menarcheal age, 13.0 years for girls born in 1988, decreased to 12.6 years for girls born in 2003 in a very recent study [7]. These findings are consistent with those of other Western countries. The estimated ages at menarche were 13.42 and 13.13 years for girls born in the 1991 and 2006 cohorts, respectively.
On the other hand, recent studies have suggested that the age at menarche is not advanced or slightly advanced compared to that of a previous report, whereas the age at breast development was observed earlier in girls evaluated in the mid-2000s [8]. The earlier timing of breast development in girls seems to be independent of the HPG axis, but it might be attributed to other potential mechanisms such as endocrine disruptors and nutritional effects. The earlier timing of breast development also affected the definition of Tanner stage, which classified breast development into 5 stages using inspection but not palpation [9]. A significant decrease in age at menarche has been observed in developing countries, which may be related to increased socioeconomic status and increased body mass index (BMI) [10]. In addition, controversy persists regarding whether it is valid to modify the definition of PP considering the relationship between early age at menarche and adverse health outcomes in adults. To date, the traditional definition of PP has been applied in clinical settings in Korea and other countries.
2. Impact of PP on health outcomes
At the time of a PP diagnosis, the main concerns are early menarche in girls, short adult stature due to early skeletal maturation, and psychosocial problems. However, the early timing of pubertal onset may increase an individual’s long-term health risks. Evidence indicates that early menarcheal age is related to an increased risk of obesity and its related diseases (hypertension and type 2 diabetes mellitus), cardiovascular disease, and mortality [11]. An early age at menarche has been suggested to increase a woman’s risk of breast cancer [12]. Early exposure to sex steroids, especially estrogen, plays a significant role in this relationship during the initial stages of breast development and throughout the reproductive years. In addition, PP can affect future psychosocial outcomes, including internalizing problems such as depression, anxiety, bulimia, and excessive psychosomatic symptoms as well as externalizing symptoms such as substance abuse, smoking, bullying, and truancy [13].
3. CPP and genetics factors
PP includes central PP (CPP, with a GnRH-dependent mechanism of development) and peripheral PP (PPP, with a GnRH-independent mechanism of development). CPP is defined as premature activation of GnRH release, whereas PPP is defined as the development of secondary sexual characteristics independent of GnRH pulsatile secretion. PPP, which is related to exogenous sex steroids, autonomous ovarian cysts, or human chorionic gonadotropin, is less frequent than CPP [14]. Approximately 80% of PP cases are estimated to be CPP [14], and CPP occurs more frequently in girls than in boys [14]. Organic brain diseases such as hypothalamic hamartoma, suprasellar arachnoid cysts, and hydrocephalus may lead to CPP, and approximately one-third of all CPP cases are considered related to organic brain lesions [15]. The cause of CPP in most girls is not identifiable and called idiopathic CPP (ICPP) [15].
Approximately 50% of boys with CPP have organic lesions in the brain [15]. However, a recent study evaluated whether boys with CPP have a lower prevalence of brain lesions than previously reported [16]. Other studies reported an increased prevalence of ICPP [17]. Genetic factors may be involved in the diagnosis of ICPP in both girls and boys. In addition, recent advances have enabled the identification of genetic predispositions in girls and boys with CPP versus those with normal puberty. Recent studies of genetics and CPP are summarized in Table 1. Therefore, this review focuses on the genetic and epigenetic factors related to CPP.
Genetic factors associated with ICPP
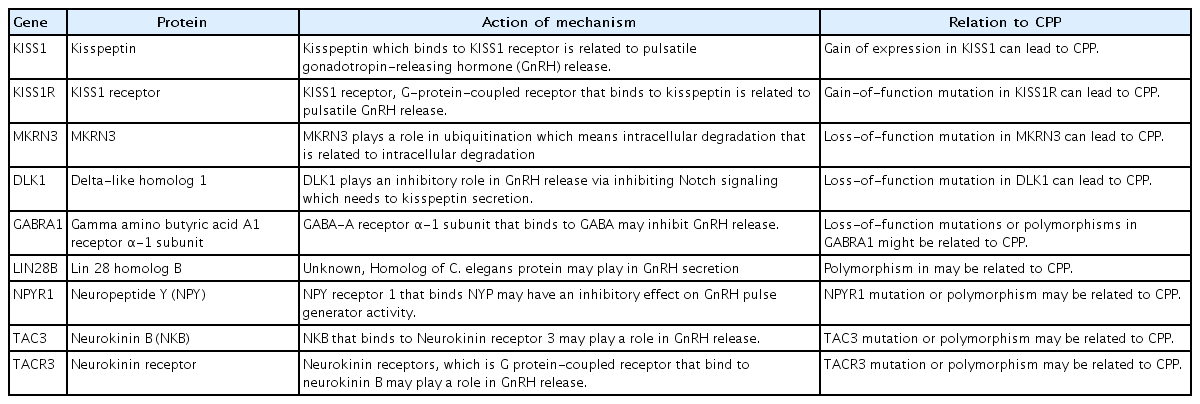
The progression of puberty is mediated by a complex control mechanism between genetic and nongenetic factors, such as environmental, nutritional, and epigenetic factors [18]. The genetic background of pubertal onset is supported by studies showing that maternal age at menarche is associated with pubertal timing in her offspring [19]. The impact of genetic factors on pubertal timing is also supported by twin studies indicating that a higher correlation for milestones of pubertal development was observed in monozygotic twins than in dizygotic twins [20]. Table 2 shows the genes related to CPP progression. Of these genes, only 4 (KISS, KISS1R, MKRN3, and DLK1) have been confirmed to play a role in patients diagnosed with CPP to date. Other genes have been implicated in ICPP, but their relationship remains to be elucidated.
1. Kisspeptin gene (KISS1)
Kisspeptin, which is encoded by the KISS1 gene, is a key factor related to pubertal onset. This peptide hormone is found in a few tissues in the human body, such as the hypothalamus, adrenal gland, and pancreas [21]. Kisspeptin acts as a ligand for the kisspeptin receptor, which is encoded by the KISS1R gene [21]. Kisspeptin is the most potent stimulator of GnRH secretion [21]. A higher level of kisspeptin augments the pulsatile release of GnRH [21]. Elevated kisspeptin levels are associated with CPP [22]. A study showed that KISS1 mutations lead to CPP, which then leads to elevated kisspeptin levels [23]. In that study, a higher kisspeptin concentration resulted from resistance to degradation. Higher kisspeptin levels were observed in girls with CPP than in agematched prepubertal controls [24]. In addition, kisspeptin levels were significantly reduced after 6 months of treatment compared to those at the start of treatment in girls with CPP [25]. A Korean study showed that genetic variations in the KISS1 gene are related to the development of CPP [26]. However, kisspeptin mutations appear to account for very few cases of CPP, as largescale studies in children with CPP failed to identify additional individuals harboring abnormalities in these genes [27]. Mutations have not been identified in girls with CPP, although a few studies have evaluated genetic alterations in the KISS1 gene [28].
2. Kisspeptin receptor (KISS1R) gene
The kisspeptin receptor, also known as G-protein-coupled receptor 54 (GPR54), has a structure similar to that of the galanin receptor [29]. The activated kisspeptin receptor, which results from the binding of its ligand, kisspeptin, recruits secondary intracellular messengers such as inositol triphosphate and diacylglycerol [30]. This process leads to the release of intracellular calcium and activation of protein kinase C [30]. A study suggested that an autosomal-dominant missense mutation in KISS1R (a gain-of-function mutation) results in activation of the intracellular signaling pathway and can be related to CPP [31]. A recent Korean study suggested that sequence variants of the KISS1R gene were related to CPP in girls [32]. However, few studies have identified mutations in these genes in girls with CPP. Although the KISS1 and KISS1R genes play a pivotal role in pubertal onset mediated by GnRH secretion, they do not seem to account for a significant number of CPP cases in children.
3. Makorin ring finger protein 3 (MKRN3) gene
The MKRN3 gene, which is located within the Prader-Willi syndrome (PWS) region on chromosome 15q11.2, is maternally imprinted (in other words, paternally expressed) and encodes MKRN3, a zinc finger protein. MKRN3 is characterized by zinc finger RING, which is found in most E3 ubiquitin ligases [33]. E3 ubiquitin ligases serve as a component of the degradation of intracellular proteins called the Ub-proteasome pathway, whereas extracellular proteins and some cell surface proteins are degraded within lysosomes [34]. MKRN3 may interact with multiple proteins related to pubertal timing, insulin signaling, RNA metabolism and cell-to-cell adhesion [35]. In a mouse model, Mkrn3 bound to neuronal pentraxin-1 (Nptx1), which plays a role in neuronal development and is highly expressed during pubertal onset [36]. Nptx1 may be degraded via polyubiquitination after interacting with the E3 ubiquitin ligase domain in Mkrn3 [36]. Highly expressed Mkrn3 is observed in the hypothalamus of mice during the neonatal and juvenile period, whereas abruptly decreased expressed Mkrn3 was observed before pubertal development [37]. Mkrn3 plays an inhibitory role in pubertal onset in mice The MKRN3 gene is believed to suppress pubertal onset in humans as well [37]. The inhibitory role of the MKRN3 gene in humans has been supported by a number of studies. A few studies have suggested that decreased serum MKRN3 levels are observed in girls and boys before pubertal onset [38]. Serum levels of MKRN3 were lower in girls with CPP versus their age-matched controls [39]. In addition, multiple variants in MKRN3, including frameshift, nonsense, and missense, which can lead to decreased MKRN3 functioning, have been suggested as linked to CPP across ethnicities and nations [40]. A meta-analysis suggested that mutations in MKRN3 were found much more frequently in familial CPP (approximately 46%) than in sporadic CPP (approximately 4%) cases, and they are considered the most common cause of familial CPP [41]. In that study, the median age at puberty onset was relatively early at 6.0 years in girls and 8.25 years in boys [41].
4. Delta-like homolog 1 (DLK1) gene
The DLK1 gene, located on chromosome 14q32.2, is maternally imprinted but paternally expressed as the MKRN3 gene [42]. This region includes imprinted genes (DLK1-DIO3 domain) associated with Kagami-Ogata syndrome, which is related to maternally expressed genes, and Temple syndrome, which is associated with paternally expressed genes [42]. The DLK1 gene has 5 exons (transcript length: 4657 bp in humans), and it encodes a transmembrane protein belonging to the epidermal growth factor–like family of proteins, which includes Notch receptors and Delta and Serrate ligands; it is part of the Delta Notch pathway, a signaling pathway conserved across species during evolution [42].
The signaling pathway in mammals has 4 Notch receptors (Notch 1-4), 5 canonical ligands (Dll1, Dll3, Dll4, Jagged1, and Jagged2) which include the Delta, Serrate, and Lag2 (DSL) domain, and 2 noncanonical ligands (Dlk1, Dlk2) [43]. Whereas canonical Notch ligands play a role in activating the Notch cascade through cell-to-cell interactions, noncanonical Notch ligands, including Dlk1, inhibit Notch signaling. Notch signaling cascades reportedly promote cell proliferation, apoptosis, and differentiation during embryonic development, and to regulate tissue homeostasis and stem cell maintenance in adults [44]. The DLK1 protein plays a role in the differentiation of neuroendocrine cells as well as osteogenesis, adipogenesis, hematopoiesis, and hepatocyte production [45]. In mice, Dlk1 is reportedly encoded in neuroendocrine tissues including the pituitary gland during fetal development [46]; it is also observed postnatally in the hypothalamus, including the medio-basal hypothalamus, a control center for GnRH secretion [47].
The formation of kisspeptin neurons in the hypothalamus requires sufficient Notch signaling in early neuronal development [48]. Notch signaling is also required for the development of kisspeptin neurons in adulthood [48]. Although the mechanism between DLK1 and pubertal timing is fully established, it is postulated that an inhibitory role of DLK1 could regulate kisspeptin neuron formation and maturation and/or kisspeptin secretion [49]. Indeed, a very recent study demonstrated a paternally expressed imprinted pattern of genomic defects in DLK1, including a 14-kb deletion and 269-bp duplication in a family with 5 female members with CPP [50]. A subsequent study reported 3 frameshift mutations in DLK1 in five women from 3 families with CPP [51]. Another study from Korea suggested 5 polymorphisms in DLK1 in 100 girls with ICPP [52]. In that study, only a polymorphism reportedly resulted in splicing defects in an in silico study, suggesting that DLK1 mutations may be a relatively rare cause of ICPP [52].
5. Gamma-aminobutyric acid receptor α1 subunit (GABRA1) gene
GABRA1, located on chromosome 5q34, encodes the gamma-aminobutyric acid (GABA)-A receptor α1 subunit, a GABA-A receptor that is a ligand-gated ion channel consisting of at least 18 different subunits (α1-α6, β1-β4, γ1-γ4, δ, π, and ρ1-ρ2) [53]. GABA, a ligand for the GABA-A receptor, reportedly plays a role in inhibitory neurotransmitters in the intrinsic mechanism of the onset of puberty in primates [54]. Of several GABA-A receptors, the α1 subunit is suggested to be related to the inhibitory activity of GABA on GnRH release. A study demonstrated that reducing tonic GABA inhibition via competitive antagonism of GABA-A receptors (bicuculine) is related to advanced pubertal onset in monkeys [55]. The GABAergic inhibition of GnRH neurons via the GABA-A receptor α1 subunit seems to be critical for repressing pubertal onset [56]. Loss-of-function mutations or polymorphisms in GABRA1 can be established to explain premature GnRH release. However, a study of the GABRA1 gene in 31 girls with ICPP found no functional GABRA1 mutations [57]. That study identified 7 GABRA1 polymorphisms in 2 exons (156T>C and 1323G>A) and 5 introns (IVS2-712(GT)n, IVS3+12A>T, IVS8+45T>G, IVS9+76A>G, and IVS10+15G>A).57) A subsequent study suggested that GABA-A receptor knockout mice exhibited normal pubertal onset and reduced amplitudes and frequencies of GABA-A postsynaptic currents [58].
6. Lin-28 homolog B (LIN28B) gene
The LIN28B gene is a human homolog of the lineage-28 (lin-28) gene of Caenorhabditis elegans that is essential for the timing of developmental events [59]. LIN28B is a highly conserved RNA-binding protein that blocks microRNAs of the LET7 microRNA family [59]. LIN28B and the LET7 microRNA family reportedly play a role in embryonic stem cell self-renewal, cell developmental and differential processes, metabolism, and oncogenesis. It may also be involved in pubertal development. A few studies have suggested that LIN28B is involved in CPP [60]. LIN28B was suggested to be related to earlier breast development, earlier menarche, and more advanced pubic hair development in a meta-analysis of genome-wide association studies [61]. Some studies reported that the rs314276 genotype in LIN28B is associated with earlier puberty or CPP [62]. Polymorphism in LIN28B, including rs7759938 and rs314280, is related to the risk of ICPP [63]. However, another study evaluated the association between variants in LIN28B and ICPP in 178 Brazilian children with CPP but did not show the causative relationship [64]. Moreover, genetic variations in LIN28B, such as rs314276, are reportedly associated with increased weight and BMI [62]. Obesity is strongly related to early pubertal development [10]. Thus, the role of the LIN28B gene in ICPP remains unclear.
7. Neuropeptide Y (NPYR) gene
Neuropeptide Y (NPY), which is highly conserved across many species, is abundant in the peripheral and central nervous systems [65]. This 36-amino-acid peptide is considered a major component in the inhibition of pulsatile GnRH secretion during the prepubertal period in nonhuman primates [65]. The central administration of NPY inhibited pulsatile GnRH release in adult female monkeys [66]. The NPY gene and its protein expression in monkeys were negatively related to GnRH pulse generator activity [67]. NPY is considered a fundamental component of pubertal onset in primates. NPY interacts with a family of G-protein coupled receptors (NYP receptors) encoding the NPYR gene located on chromosome 4q, which includes the Y1, Y2, Y4, Y5, and Y6 subtypes [68]. Of them, the Y1R subtype reportedly regulates hormone secretion and is implicated in the effects of NYP on GnRH release [69]. A causal relationship was observed between mutations or polymorphisms in the NPYR gene and ICPP [70]. A Brazilian study attempted to evaluate NPYR1 mutations or polymorphisms in 33 girls with familial ICPP [71]. Although a heterozygous synonymous polymorphism (K374T) was found, it did not result in amino acid substitution and was detected in the control population at a high prevalence (28%) [71]. In addition, they did not detect altered polymorphisms via in vitro assays [71].
8. Tachykinin 3 (TAC3) and tachykinin receptor 3 (TACR3) genes
TAC3 and TACR3 genes encode NKB and the G protein-coupled receptor neurokinin (NK3R) [72]. NKB is a mammalian tachykinin family of peptides such as substance P (SP), neurokinin A (NKA), NKB, neuropeptide K, and neuropeptide Y (NPY) [72]. These tachykinins reportedly mediate nonadrenergic and noncholinergic excitatory neurotransmissions [72]. There are 3 main classes of neurokinin receptors, including NK1R (SP preferring receptor), NK2R (NKA preferring receptor), and NK3R (NKB preferring receptor). NKB and NK3R are co-expressed with KNDy neurons [73]. The NKB system has been implicated as a regulator of human reproduction. In a primate study, an NKB or NKB analog (senktide) led to robust GnRH secretion [74]. Loss-of-function mutations in the TAC3 or TACR3 genes were identified in subjects with familial hypogonadotropic hypogonadism, suggesting that the NKB and NK3R pathways are indispensable components of pubertal onset [75]. In addition, a mutation in the TACR3 gene was identified in a patient with ICPP [76]. Other studies revealed variants in TAC3 or TACR3 genes in patients with ICPP [77]. However, the association between variants or mutations in the TAC3 or TACR3 genes and ICPP has yet to be confirmed, and functional assays for this mutation are needed.
Nongenetic factors associated with ICPP
1. Environmental and socioeconomic factors
Variations in pubertal onset are related to environmental factors, such as nutrition, intrauterine conditions, and exposure to endocrine-disrupting chemicals (EDCs) [18]. Interactions with the environment and hypothalamic signals are thought contribute to the downward secular trend of pubertal timing in developed and developing countries. Several studies have shown the trends of earlier timing of pubertal development, including the Republic of Korea [78]. Obesity due to improved nutrition is thought to be strongly linked to the downward secular trend in the timing of puberty and these differences. On dual-energy x-ray absorptiometry and according to estimated BMI, an earlier age at menarche was related to an increased body fat mass due to an improved diet [79]. Intrauterine growth restriction or small for gestational age (SGA) status is reportedly related to earlier age at pubertal development in the general population [80] as well as CPP in patients with Silver-Russel syndrome [81]. In addition, EDCs are known to be responsible for earlier pubertal onset. However, the mechanism between idiopathic CPP and nongenetic environmental factors remains unclear. These relationships are suspected to be mediated by genetic effects [82].
2. Epigenetic factors
It seems reasonable that puberty onset is regulated as an alternative pathway. It has been suggested that the epigenetic modification of gene expression, specifically heritable changes in gene expression that occur without changing a gene’s primary nucleotide sequence, may be an alternative pathway [83]. Modes of epigenetic regulation involve DNA methylation, histone posttranslational modifications, and noncoding ribonucleic acids (miRNAs) [84]. Gene-specific gatekeeper functions and gene expression plasticity are believed regulated by epigenetic mechanisms [85]. Epigenetic information is reportedly involved in sexual differentiation of the brain [86]. The epigenetic mechanism of transcriptional repression in the hypothalamus reportedly played a role in the timing of pubertal onset in a rat model [87].
The polycomb group (PcG) complex is considered a major contributor to this repressive mechanism. Expression of the PcG complex in the hypothalamus is decreased at puberty, which is associated with the acquisition of epigenetic silencing marks such as DNA methylation and histone modification [88]. In brief, PcG of transcriptional repressors prevent the premature initiation of puberty by inhibiting Kiss1 transcription in KNDy neurons [89]. Although Kiss1 expression has been suggested to be inhibited in the prepubertal period, the promoters of PcG complexes become methylated at the timing of pubertal onset, allowing other epigenetic modifications such as epigenetic activators to turn on the switch of puberty [88]. Changes in chromatin status due to the separation of PcG components from the promoter reportedly result in increased epigenetic marks, allowing gene activation. These changes can lead to elevated mRNA expression in Kiss1 [90] cell. The Kiss1 promoter, in which repressing and activating marks coexist, is also subjected to increasing epigenetic-activating marks [91]. Elevated levels of an activating transcriptional complex can be observed along with the loss of PcG inhibition [91].
The trithorax group (TrxG) complex is suggested to play a role as a promoter and enhancer in the Kiss1 gene [91]. The TrxG complex may assist in driving out PcG by demethylating repressive histones, increasing an active enhancer. By implementing the TrxG, an epigenetic modification is suggested to change the Kiss1 gene from a repressed to active state at the timing of pubertal onset [91]. However, the epigenetic mechanism of ICPP in human remains unclear.
A possible mechanism between epigenetics and pubertal onset may be found in the MKRN3 gene in humans. The gene is expressed by only one of the inherited alleles and may be fragile to genetic mutations and/or epigenetic modifications in the coding and regulating regions. For PWS, the MKRN3 gene is activated from a paternally imprinted region [92]. Mutations in the promoter region of MKRN3 are suggested to alter Mkrn3 expression by disrupting the binding of specific transcription factors [93]. Altered methylation at the MKRN3 gene is postulated to be involved in pubertal onset. However, a previous study suggested a possible mechanism between changes in methylation and pubertal onset [94]. Zinc finger protein 57 (ZFP57), a C2H2 zinc finger protein that contains a repressive Krüppel-associated box (KRAB) domain [95], is considered to regulate genomic imprinting, including MKRN3 [96]. Hypomethylation of the promoter region of ZFP57 was identified in the blood cells of pubertal girls, while elevated ZFP57 expression was observed in the hypothalamus of female monkeys at pubertal onset. In addition, KRAB-associating protein (KAP1) is a ZFP57-cofactor and considered to play a role in DNA methylation. Knockout of the ZFP57 cofactor is related to elevated hippocampal Mkrn3 expression [97]. Regulation of the ZFP57 and KAP1 genes may alter chromatin structure and regulate transcription, including imprinted regions [98]. It is possible that elevated ZFP57 levels mediate the alteration of MKRN3 chromatin to facilitate its repression and allow pubertal onset [99], although this has yet to be confirmed.
Conclusion
Pubertal onset seems to be minutely controlled by interactions between genetic and nongenetic factors. ICPP is a result of the premature activation of these interactions. Recently identified genetic factors aid our understanding of the early development of puberty. The KISS1, KISS1R, MKRN3, and DLK1 genes contribute to the established genetic factors related to ICPP, whereas the GABRA1, LIN28B, NPYR, TAC3, and TACR3 genes are considered possibly related to CPP [18,23,31,39,51]. Environmental and socioeconomic factors, such as nutrition, SGA, and EDCs, may also be involved in ICPP. Epigenetic control may mediate the relationship between genetic factors and environmental and socioeconomic factors. A variety of studies are still needed to validate the mysterious pathophysiology of puberty regulation as new factors continue to emerge. Comprehensive and step-by-step research of genetic and nongenetic factors can increase our understanding of the exact mechanism of ICPP.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.