Four months of rifampicin monotherapy for latent tuberculosis infection in children
Article information
Abstract
Diagnosing and treating latent tuberculosis infection (LTBI) is an important part of efforts to combat tuberculosis (TB). The Korean guidelines for TB published in 2020 recommend 2 LTBI regimens for children and adolescents: 9 months of daily isoniazid (9H) and 3 months of daily isoniazid plus rifampicin. Isoniazid for 6–12 months has been used to effectively treat LTBI in children for over 50 years. However, a long treatment period results in poor patient compliance. This review summarizes pediatric data on the treatment completion rate, safety, and efficacy of 4 months of daily rifampicin (4R) and evaluates the pharmacokinetics and pharmacodynamics of rifampicin in children. The 4R regimen has a higher treatment completion rate than the 9H regimen and equivalent safety in children. The efficacy of preventing TB is also consistent with that of 9H when summarizing reports published to date. A shorter treatment period could increase patient compliance and, therefore, prevent TB in more patients. By using an effective, safe, and highly compliant regimen for the treatment of children with LTBI, we would become one step closer to our goal of eradicating TB.
Key message
· Recently, the importance of a short-term treatment regimen including rifamycin has been highlighted in the treatment of latent tuberculosis infection (LTBI).
· Four prospective or retrospective studies in children consistently reported that a 4-month daily rifampicin regimen (4R) had a higher completion rate than and comparable safety to a nine-month daily isoniazid regimen.
· We suggest rifampicin 20–30 mg/kg/day for children aged 0–2 years and 15–20 mg/kg/day for children aged 2–10 years in 4R to treat LTBI.
Introduction
Diagnosing and treating latent tuberculosis infection (LTBI) is an important part of the effort to combat tuberculosis (TB). Worldwide, an estimated 1.7 billion people, or about a quarter of the population, are infected with Mycobacterium tuberculosis [1]. The prevalence of TB infection estimated by the tuberculin skin test (TST) in boys (girls) aged 10–14 years in Korea was 74.5% (67.9%) in 1965 and decreased to 16.5% (16.9%) by 1995 [2]. In 2016, when the prevalence of LTBI among Korean citizens aged 10–64 years was investigated, the TST-positive rate of all subjects was 33.2%, while that of the population aged 10–19 years was 6.5% [3]. The management of LTBI in Korea is gradually expanding and comprises an essential element of the national TB control plan [4,5]. In 2004, LTBI examinations were conducted for family contacts aged <6 years. In 2008, the contact screening age was expanded to <18 years. Since 2013, contact screenings have included families as well as daycare centers, kindergartens, and schools to identify and treat infected children and adolescents [4,6]. Since 2015, the Korean government has covered the cost of treatment for all citizens with LTBI [6].
The 4th edition of the Korean Guidelines for Tuberculosis published in 2020 recommends 2 regimens for treating LTBI in children and adolescents: 9 months of daily isoniazid (9H) and 3 months of daily isoniazid plus rifampicin (3HR) [7]. Traditionally, rifampicin monotherapy was primarily used only when isoniazid was not available [8]. However, in recent years, rifamycin-based regimens, which feature a shorter treatment duration than 9H, are recommended to increase the treatment completion rate. In 2020, the Centers for Disease Control and Prevention (CDC) recommended 3 months of once-weekly isoniazid plus rifapentine (3HP) for children aged >2 years and 4 months of daily rifampicin (4R) for children of all ages. A regimen of 3 months of daily isoniazid plus rifampicin was conditionally recommended for children of all ages [9]. Among these drugs, rifapentine is currently not available in Korea.
LTBI treatment in children began in 1954 by Dr. Lincoln [10]. She found that isoniazid administration prevented meningeal TB in children with asymptomatic primary TB. The first placebo-controlled study of rifampicin monotherapy was conducted in Hong Kong in patients with silicosis. This study compared 4 groups: daily isoniazid for 6 months, 3HR, 3 months of daily rifampicin (3R), and placebo. The results showed no difference between the 3 treatment regimens for TB disease prevention, although the incidence of TB disease and hepatotoxicity was lowest in the group receiving 3R [11]. The American Thoracic Society recommended 4R as an alternative regimen in 2000. Subsequent randomized trials in adults consistently demonstrated better treatment completion rates, with significantly fewer adverse events, particularly grade 3–4 liver toxicity, than 9H [8,9,12].
This review summarizes pediatric data on the treatment completion rate, safety, and efficacy of 4R and evaluates the pharmacokinetics and pharmacodynamics of rifampicin in children and the recent safety issues associated with rifampicin.
Characteristics of rifampicin for treating TB in children
1. Antibacterial activity
Rifampicin was developed in 1965 by Dow-Lepetit Research Laboratories (Milan, Italy) [13]. It is a semisynthetic derivative of rifamycin b, which inhibits bacterial RNA synthesis by binding to the beta subunit of DNA-dependent RNA polymerase, interfering with RNA transcription, and eventually inhibiting protein synthesis [14]. Rifampicin exerts antibacterial activity against gram-positive and -negative bacteria, intracellular pathogens (including Chlamydia, Legionella, Brucella, and Bartonella spp.), nontuberculous mycobacteria, and M. tuberculosis [15]. Rifampicin exhibits bactericidal ability against actively dividing bacteria as well as those that occasionally metabolize for a short period, killing them more rapidly than isoniazid [16].
Rifampicin was first used clinically in Italy in 1968 and has been the most important drug for the treatment of TB for approximately 50 years. Rifampicin in combination with anti-TB drugs has reduced the overall treatment duration [17].
2. Pharmacokinetics and pharmacodynamics of rifampicin
Rifampicin is well absorbed when taken orally and rapidly absorbed in the intestine during fasting but less so when taken with food. It is well distributed in most tissues and body fluids. As it is fat-soluble, it passes through the blood-brain barrier. It is metabolized in the liver and has a half-life of 3–4 hours. Since the half-life may be prolonged in patients with abnormal liver function, dose adjustment is necessary in patients with liver failure. However, no dose adjustment is required in patients with renal insufficiency, as only 13%–24% is excreted in the urine [15,18].
The bactericidal ability of rifampicin against M. tuberculosis depends on its concentration, and the most correlated pharmacokinetic/pharmacodynamic parameter is the area under the concentration-time curve to minimum inhibitory concentration (MIC) ratio. Preventing the emergence of resistance is associated with the free peak concentration (C(max)) to MIC ratio [19].
3. Rifampicin dose for treating pediatric TB
When using 4R for the treatment of LTBI in adults, the recommended dose of rifampicin is 10 mg/kg. However, when children aged 3 months to 13 years (mean, 4 years) were administered a mean rifampicin dosage of 9.6 mg/kg, they showed very low serum concentrations, with a mean maximum concentration (Cmax) of 4.9–6.9 μg/mL [20]. The desirable Cmax of rifampicin suggested in healthy adults is 8–24 μg/mL. It is recommended that, if the Cmax is 6 or less, the dose of rifampicin should be increased [21]. In 2010, the World Health Organization (WHO) increased the dose of primary anti-TB drugs in children to improve treatment outcomes and recommended rifampicin doses of 15 mg/kg (range, 10–20 mg/kg; maximum dose, 600 mg/day) [22]. After the guideline change, one study compared rifampicin 10 mg/kg to 15 mg/kg in 11 children under 2 years of age. When rifampicin was administered at 10 mg/kg, the mean Cmax was 6.36 μg/mL (4.45–8.27 μg/mL), whereas at 15 mg/kg, it was 11.7 μg/mL (8.7–14.7 μg/mL), thus reaching an appropriate therapeutic concentration with the latter [23]. However, in one study in which children aged <10 years (median age, 2.29 years) were given rifampicin 9–22 mg/kg, only 2/31 (6%) of children attained the therapeutic concentration [24]. Moreover, in 2016, Bekker et al. [25] reported that when 39 infants aged <12 months (mean age, 6.6 months) were administered rifampicin 10.1–20.5 mg/kg, the mean Cmax was 2.9 μg/mL, much lower than expected. In this study, 2 rifampicin formulations were used in which infants receiving a rifampicin formulation administered at a lower dose had higher mean rifampicin concentrations than those receiving a higher dose. Thus, at least in this study, dose alone was not associated with Cmax.
In another study of 62 children with a median age of 5 years diagnosed with active TB, the children received a median dose of 16 mg/kg (interquartile range [IQR], 13.8–19.8 mg/kg) of rifampicin. The median Cmax of rifampicin in the subjects was 6.3 μg/mL (IQR, 3.5–8.8 μg/mL); among the 51 subjects who received the dose range recommended by WHO, i.e., 15 mg/kg (IQR, 10–20 mg/kg), only 21 (41.2%) reached the target concentration [26]. Aruldhas et al. [27] developed a population pharmacokinetic model using concentration-time data of rifampicin from 41 children aged 2–16 years diagnosed with pulmonary or lymph node TB. In a simulation using this model, 28.8% of children weighing 4–39 kg had a Cmax greater than 8 μg/mL after rifampicin 10.7- to 18.7-mg/kg administration. However, when rifampicin 35–40 mg/kg was administered to children weighing 6–30 kg, 74.2% had a Cmax greater than 8 μg/mL.
In a pediatric study, children with low blood levels of rifampicin were more likely to have poor treatment outcomes, and exposure to rifampicin was the lowest in children with a low body weight or human immunodeficiency virus (HIV) coinfection [28]. Another study of 113 pediatric TB patients in Ghana reported that HIV-infected pediatric TB patients had lower exposure to rifampicin, i.e., a lower Cmax and a lower area under the concentration-time curve from 0 to 8 hours (area under the curve=0–8) than children with TB alone [29].
Other factors involved in the bioavailability of rifampicin include the characteristics of the raw materials, additives of formulations, diversity of manufacturing processes, degradation in the gastrointestinal tract, and inherent variability in drug absorption and metabolism [30].
Based on the above-described results, the recommended dose of rifampicin in the treatment of LTBI has increased to achieve blood concentrations above the MIC of M. tuberculosis in HIV-infected and non-HIV-infected children. The 2018 American Academy of Pediatrics (AAP) guidelines increased the daily dose of rifampicin to 15–20 mg/kg for treating TB in children aged 0–15 years. The guidelines also added that many experts recommend rifampicin 20–30 mg/kg/day for the treatment of TB in infants and toddlers (i.e., 0–2 years) and children of any age with severe TB [31]. In 2020, the CDC guidelines for LTBI recommended that children (aged 2–17 years) receive rifampicin 15–20 mg/kg for 4R and that infants and toddlers (aged 0–2 years) receive 20–30 mg/kg while referring to the AAP guidelines in the footnotes [9]. The WHO guideline published in 2020 recommends rifampicin 10 mg/kg/day for the 4R regimen for children aged >10 years and rifampicin 15 mg/kg/day (range, 10–20 mg/kg/day) for children aged <10 years [32]. Considering the relationship between the administered dose of rifampicin and its serum concentrations in previous studies, it may not be possible to achieve an adequate blood concentration of rifampicin in very young children at the dose recommended by the WHO. We suggest that very young children (age, 0–2 years) be given rifampicin 20–30 mg/kg and children aged 2–10 be given rifampicin 15–20 mg/kg.
4. Adverse effects
Rifampicin is an anti-TB drug with relatively few major adverse effects. Its toxicity can be largely divided into 2 types: hepatotoxicity and immunoallergic effects. Hepatotoxicity due to rifampicin has a much lower incidence than that caused by isoniazid [33,34]. Administering rifampicin resulted in a transient elevation of liver enzyme levels within the first few weeks in 10%–20% of patients, but in most cases no special measures were required [35-37]. Although rifampicin alone has a low risk of hepatotoxicity, a review published in 1991 reported an increased incidence of clinical hepatitis when isoniazid and rifampicin were administered to children compared with isoniazid alone. Although few studies have reported very high rates of clinical hepatitis, children receiving isoniazid and rifampicin were 4 times more likely to develop hepatitis than those receiving isoniazid alone [33]. However, the mechanism of this phenomenon is unknown.
The immunoallergic effects associated with intermittent therapy range from minor (skin, digestive system, or flu-like syndrome) to major (hemolytic anemia, shock, or acute kidney injury) [36,38]. When rifampicin is administered daily, the rash usually appears at the beginning of treatment; most reactions are mild and transient and improve with observation without drug discontinuation [36,39]. Flu-like syndrome rarely occurs with daily rifampicin therapy, but it can be seen with intermittent dosing, especially once-weekly dosing [36]. Gastrointestinal symptoms may include anorexia, nausea, mild abdominal pain, and vomiting, but most are mild. Gastrointestinal symptoms are also more common in patients receiving intermittent therapy than in those receiving daily therapy [40]. Hematological adverse events are rare, and rifampicin-induced thrombocytopenia is associated with antiplatelet antibodies and improves upon drug discontinuation [41].
5. Drug interactions
Rifampicin induces cytochrome P450 (CYP450), drug transport protein expression, and drug-metabolizing enzymes such as uridine 5'-diphospho-glucuronyltransferases and sulfotransferases [42,43]. Drugs such as oral anticoagulants, antifungal drugs, and oral contraceptives, although not used frequently in children, interact with rifampicin. In addition, since rifampicin is a potent CYIP3A4 inducer, it reduces plasma concentrations of the nonnucleoside reverse transcriptase inhibitor and the protease inhibitor, CYP3A4 substrates, a problem in people receiving rifampicin for HIV-associated or preventive TB treatment [42,44]. CYP3A4 inducers may decrease serum concentrations of corticosteroids such as dexamethasone and methylprednisolone. Therefore, when prescribing dexamethasone or prednisolone to patients receiving rifampicin, it is necessary to consider increasing the corticosteroid dose and closely monitoring whether the corticosteroid efficacy decreases [43].
Rifampicin is also a potent inducer of CYP1A2 and CYP2C19 and an intermediate inducer of CYP2B6, CYP2C8, and CYP2C9. Therefore, antiretroviral agents that are metabolized by one of these enzymes are also affected [45]. Since there have been few evaluations of the efficacy of 4R in patients with LTBI and HIV coinfection, 4R is recommended for patients without HIV infection [9]. However, a recent study in adult HIV patients reported that 4R was safe and effective even in HIV-infected patients [46].
Completion rate, safety, and efficacy of 4R versus 9H for treating LTBI in children
1. Completion rate
Since the late 1950s, isoniazid for 6–12 months has been used to treat LTBI in children [47]. In 2000, American Thoracic Society recommended 9H for children and adolescents as the preferred regimen based on the results that 12 months of isoniazid was more effective than 6 months of isoniazid and that the maximal beneficial effect of isoniazid is achieved by 9 months in HIV-uninfected adults [8]. Since the Korean guidelines for TB were first published in 2011, 9H has been among the main regimens for treating TB in children. As such, 9H has long been used effectively for treating LTBI in children, but the completion rate is low due to the long administration period [47]. In children, the completion rate of 9H was approximately 50%, especially low among self-administration cases [48,49]. The program for immigrant children reported a lower completion rate, with a 68% initiation rate but only 12% completion rate [50]. Even in adults, the completion rate of 9H was reportedly only 46.5%–50% [51,52]. Very limited data are available about the completion rate of LTBI in Korean children. In one institutional study, 13 of 15 patients prescribed 9H at the beginning of treatment were reportedly completed it [53].
We searched the MEDLINE, Embase, Web of Science, and Cochrane databases to identify studies that compared completion rates and safety between 4R and 9H in children. One randomized controlled trial (RCT) and 3 retrospective studies compared the 4R and 9H completion rates children (Table 1). In the RCT, 4R had a significantly higher completion rate than 9H (Table 1) [54]. In the retrospective studies, when self-administered, 4R showed a better completion rate than 9H (93% vs. 62%) [55]. The authors conducted another study comparing 9H, 3HP, and 4R; in that study, the completion rate of 4R was higher than that of 9H when self-administered (83.5% vs. 52.6%) [56].
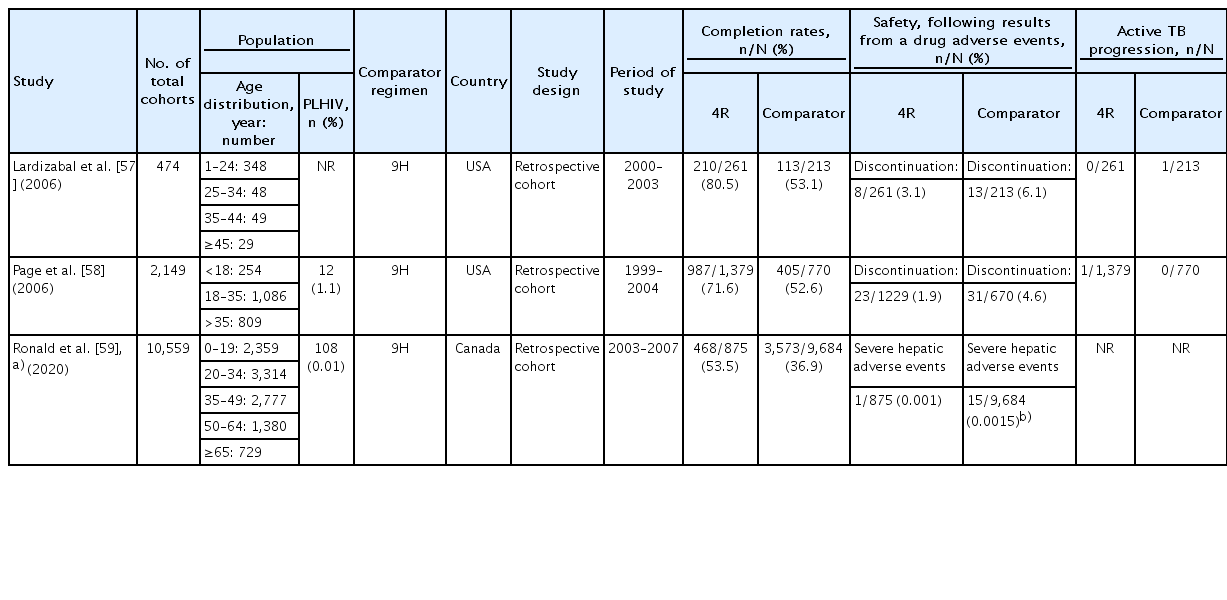
Three prospective or retrospective cohort studies compared 4R and 9H, including adults and children, among the study subjects (Table 2) [57-59]. These studies consistently reported that 4R had a higher completion rate than 9H.
2. Safety
Children are generally more tolerant of anti-TB drugs than adults. Although there are other factors contributing to this phenomenon, it may be in part because drug levels (exposure) are actually lower, as they were often underdosed in these older studies. There are few reports on drug discontinuation due to fatal hepatotoxicity or side effects during the treatment of LTBI in children. In a recent RCT comparing the safety of 4R and 9H in children, there were no cases of drug discontinuation due to adverse reactions in either group. The number of patients complaining of minor adverse events did not differ between groups (Table 1) [54]. In a cohort study of 4R, 9H, and 3HP regimens, adverse events were more common in the 9H treatment group than in the 3HP or 4R treatment groups. The most common adverse reaction was abdominal pain, and only 2 of 667 patients (0.3%) had elevated liver enzyme levels. One patient in the 9H group had an increased liver enzyme level of ≥1,000 U/L after taking the medicine for 8 months [56]. A retrospective study compared 4R and 9H and reported no intergroup difference in the incidence of adverse events. Hepatitis occurred in 0.5% of 404 patients [55]. In the study reported by Gaensbauer et al. [60] comparing 4R and 9H, the proportions of drug discontinuation cases due to adverse drug reactions were 1.5% in 4R and 0.7% in 9H, and no significant intergroup difference was observed. There were no cases of symptomatic hepatotoxicity in either group.
3. Efficacy of 4R versus 9H
LTBI treatment began with isoniazid monotherapy, and it was efficacious at preventing 70%–90% of active TB cases in children. When the drug was taken consistently, the treatment efficacy was reportedly >90% [61-63]. To confirm whether the LTBI treatment was efficacious and prevented the progression to TB disease in patients with LTBI, it is necessary to conduct a follow-up observation for a certain period after treatment. In this regard, Diallo et al. [54] followed up patients for 16 months after enrollment. During the observation period, there were no cases of TB in the 4R treatment group versus 2 cases in the 9H treatment group. The remaining studies listed in Table 1 were retrospective and observational; hence, it was difficult to confirm the treatment efficacy. However, there are a few reports of cases progressing to active TB, and no intergroup difference was confirmed. Rifampicin monotherapy was not inferior to 9H in adult studies [64]. In a network meta-analysis conducted to confirm the effectiveness of various LTBI treatment regimens, the daily administration of rifampicin for 3 or 4 months (odds ratio [OR], 0.25; 95% credible interval [CrI], 0.11–0.57) was confirmed effective at preventing TB versus no treatment [65]. Also, 9H (OR, 0.46 [95% CrI, 0.22–0.95]) and 3 or 4 months of daily isoniazid plus rifampicin (OR, 0.33 [95% CrI, 0.20–0.54]) appeared efficacious at reducing active TB versus no treatment.
Cost-effectiveness of 4R
In Korea, isoniazid costs 15 won (Korean won [KRW]; 0.01 United State dollar [USD]) per 100 mg tablet, while rifampicin costs 104 KRW (0.09 USD) per 150-mg tablet. According to the Korean guidelines for TB, daily therapy for LTBI in children requires 10 mg/kg of body weight for isoniazid and 15 mg/kg of body weight for rifampicin. Therefore, for the treatment of LTBI in a child weighing 10 kg, the cost is 4,050 KRW (3.54 USD) for 9H and 12,480 KRW (10.89 USD) for 4R. In terms of drug price alone, 4R costs more, but neither regimen is burdensome in Korea. In children, blood tests are generally not routinely performed during LTBI treatment, and drug-related side effects rarely occur during treatment. During LTBI treatment, a monthly hospital visit for clinical observation of adverse drug reactions is recommended [6]. According to this recommendation, after the initiation of treatment, 9H requires 9 hospital visits and 4R requires 4 hospital visits. Considering all healthcare costs, including the drug price, all testing, and follow-up visits, 4R will cost less. Bastos et al. [66] reported that 4R was less expensive than 9H in high-income, middle-income, and African countries.
Rifampicin susceptibility to rifampicin after rifampicin monotherapy for LTBI
Rifampicin is among the most important drugs for the treatment of TB. However, resistance could be a problem if patients progress to TB disease after LTBI treatment with rifampicin alone. Although there are few reports on this issue, a systematic review concluded that there is no evidence indicating that more rifampicin-resistant M. tuberculosis is detected in patients who develop active TB after preventive treatment with a rifamycin-based regimen [67]. In a study by Page et al. [58], 1 of 1,379 people prescribed 4R was diagnosed with cervical lymphadenitis 1 year later, and the M. tuberculosis isolate was susceptible to rifampicin. In another study of 679 patients with silicosis in Hong Kong, 15 treated with rifampicin alone during a 5-year follow-up period progressed to active TB disease, and all 15 M. tuberculosis isolates were susceptible to rifampicin [11].
Nitrosamine contaminants in rifampicin
In August 2020, the U.S. Food and Drug Administration announced that nitrosamine impurities were detected and investigated in rifampicin and rifapentine sold in the United States [68]. In fact, the 1-methyl-4-nitrosopiperazine (MNP) detected in rifampicin belongs to the nitrosamine class of compounds, some of which are classified as possible carcinogens in humans based on carcinogenicity studies in rodents. Accordingly, some experts have recommended that children receive only 9H for latent TB treatment until additional data are available [69]. In all products distributed in Korea, the MNP content of rifampicin distributed in Korea was 1.68–6.07 ppm, higher than the provisional management standard (0.16 ppm) [70]. In the United States, when the MNP content in rifampicin is <5 ppm, distribution is temporarily allowed to prevent a shortage of drugs essential for TB treatment. In Korea, the Ministry of Food and Drug Safety also allowed the distribution of rifampicin to consider the following factors. Rifampicin is an essential drug for the treatment of TB, which can be life-threatening, alternative drugs are lacking; the health effect is not significant considering the results of human impact evaluation; and its distribution is allowed in other countries. Manufacturers are trying to remove nitrosamine impurities and meet the standards of the Ministry of Food and Drug Safety. Once these standards are satisfied, then 4R can be safely administered again [70].
Conclusions
The 9H regimen has been used effectively for the treatment of LTBI in children for over 20 years. However, patient compliance was poor due to the long treatment duration. According to a recent randomized trial of treating LTBI in children, the 4R regimen had a higher completion rate than the 9H regimen with comparable safety in children. Its efficacy at preventing TB was also similar to that of 9H when reports published to date were summarized. A shorter treatment duration will increase patient compliance, and through greater compliance, it is possible to prevent progression to TB disease in more patients. This will be an important element of effort to bring us closer to our goal of eradicating TB.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.