Clinical importance of immunonutrition in infants: a review of the recent literature
Article information
Abstract
During the last decades, the role of nutrition has been well elucidated in medicine, especially among critically ill infants and children. Many nutrients have the potential to modulate the immune system. A healthy immune system is essential for the prevention and recovery of many pediatric illnesses. Intervention using specific nutrients for the immune system is called immunonutrition. Immunonutrient supplementation has been attempted to modulate inflammatory or immune responses, leading to an improved clinical course of critically ill patients with prolonged nutritional supplementation parenterally or enterally. This review discusses immunomodulatory nutrients for infants based on the recent literature.
Key message
Nutrients are important in the developing immune system. Human milk supplies diverse bioactives to prevent acute infection or chronic inflammation. Immunoglobulins, lactoferrin, and glutamine in human milk decrease gastrointestinal and respiratory infection. Human milk oligosaccharides promote the growth of intestinal microbiota, the gut barrier, and antimicrobial or antiviral activity. Micronutrients act as anti-inflammatory immunonutrients, too. However, the toxicity of some nutrients from an overdose should be considered.
Graphical abstract
Introduction
Nutrition is essential in pediatric growth and development. It also plays a pivotal role in preventing and improving outcomes of diverse diseases in pediatric patients by maintaining an optimal immune response [1]. Nutrition and the immune system are closely correlated. Nutritional intervention that has a direct or indirect immunologic effect is called immunonutrition [2]. The concept of immunonutrition differs from that of folklore medicines or foods based on individual experiences and religious faiths that are not proven via the scientific process [3].
The immune system is immature at birth, but passive immunity transmitted from the mother through the placenta and the breastmilk can protect against infection in the neonatal period [4]. Negative nutritional alterations during the prenatal or neonatal period can lead to immunologic changes, increasing the risk of infection and chronic inflammatory disease. Early-life nutritional exposure is a significant determinant of immune health [5]. The present review aimed to discuss the immunological role of several nutrients for infants to prevent infectious diseases or non-communicable diseases later in life based on the recent scientific literature.
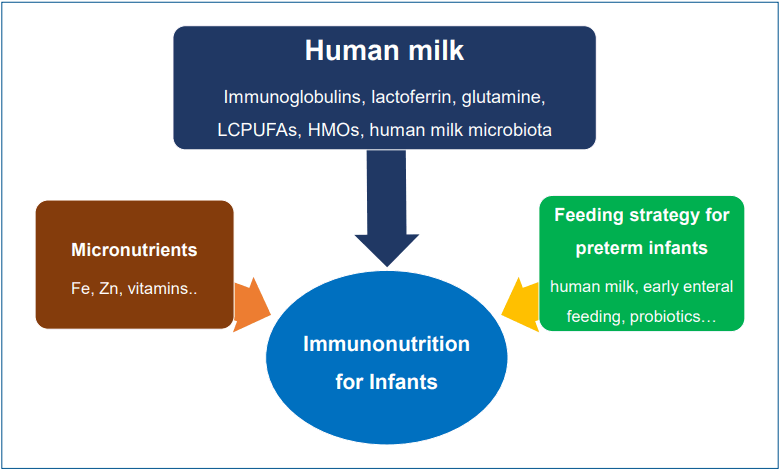
Human milk
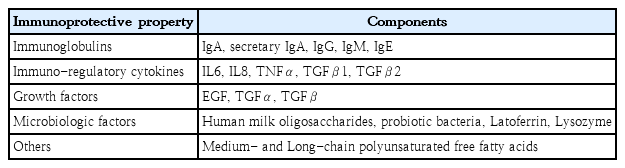
Human milk, the first food, delivers diverse nutritional and immunologic factors to neonates that contribute to development of the immune system [4]. As an optimal and exclusive nutritional source, it is recommended for all infants from birth to 6 months of age [6]. Human milk contains immunologic, protective, and trophic components such as immunoglobulins, immune-regulatory cytokines, microbiologic factors, and diverse cell types (Table 1) [3,7]. The immunologic and diverse components of human milk support the immature immune system in neonates and feature many immunonutritive benefits for infants [8]. Breastfeeding, especially the duration of exclusive breastfeeding, is related with decreased gastrointestinal and respiratory infections in early life [9-11]. It can also promote optimal health outcomes in later life [12].
1. Immunoglobulins
Immunoglobulin (Ig) A, IgG, IgM, and IgE are found in human milk. The most abundant Ig is secretary IgA (sIgA), followed by secretary IgG. SIgA in human milk is not fully digested by gastric acid in preterm or term infants. It provides lower gastrointestinal immunity and prevents the binding of toxins, bacteria, and viruses to the intestinal mucosa by neutralizing them [13,14]. Although sIgA in human milk decreases over time, its production in breastfed infants increases over the first 6 months of life [15,16]. IgG is the main immunoglobulin in the blood. It is also present in human milk, albeit at low levels [17]. However, IgG levels tend to increase in human milk as breastfeeding continues. Several studies suggest the possibility of IgG in human milk as a protective role in decreasing infections in breastfed infants [12,17,18]. Human milk can transfer IgM to infants. Term infants can partially digest IgM, whereas preterm infants cannot [13]. Human milk also contains allergen-specific IgG and IgE that may help sensitize infants [19].
2. Lactoferrin
Lactoferrin is a rich whey protein in human milk that binds well with iron. Lactoferrin in human milk is known to prevent bacterial, fungal, and viral infections. As iron is essential for microorganism growth, the iron-binding affinity of lactoferrin may inhibit bacterial growth by competing with bacteria to bind iron. However, the reported protective effects of recombinant or bovine lactoferrin are conflicting [20,21].
3. Glutamine
Glutamine, which is abundant in human milk, has been considered a fuel for rapidly dividing enterocytes and immune cells. It is a conditionally essential amino acid in critically ill patients, especially preterm in infants [20,22]. A blinded randomized study reported that glutamine supplementation for 1 month can reduce the rate of sepsis in preterm infants by blunting HLA-DR+ and CD16+ T lymphocytes [23]. Glutamine has been theoretically considered to prevent necrotizing enterocolitis (NEC), but recent well-designed randomized controlled trials have not proven the benefit of glutamine supplementation for reducing NEC rates [8].
4. Long-chain polyunsaturated fatty acids
Human milk contains a considerable amount of long-chain polyunsaturated fatty acids (LCPUFAs). Linoleic acid (18:2n-6) and alpha-linolenic acid (18:3n-3) are essential LCPUFAs that can modulate diverse immune responses in the body via lipoxins, prostaglandins, and thromboxanes [24]. LCPUFA supplementation can significantly reduce bronchopulmonary dysplasia and NEC in preterm infants by downregulating platelet-activating factor (PAF) and PAF receptor, and by downregulating endotoxin translocation into the systemic circulation [20,24]. It can also help develop retinal and cognitive function in infants [25,26]. The mean levels of arachidonic acid (AA) and docosahexaenoic acid (DHA) in human milk are about 0.5% and 0.3% of total fatty acids, respectively, but the ratio of AA/DHA in mature human milk is considerably variable depending on a mother’s diet and region [27]. Both DHA and AA are included in infant formula at amounts at least equal to DHA in human milk [27]. However, the recommended dosages or ratios remain unclear.
5. Human milk oligosaccharides
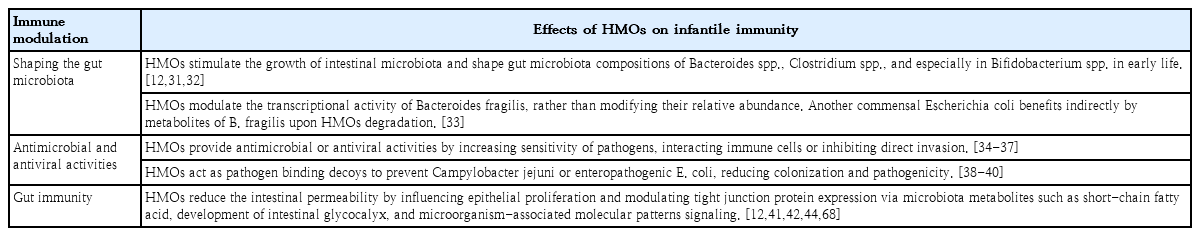
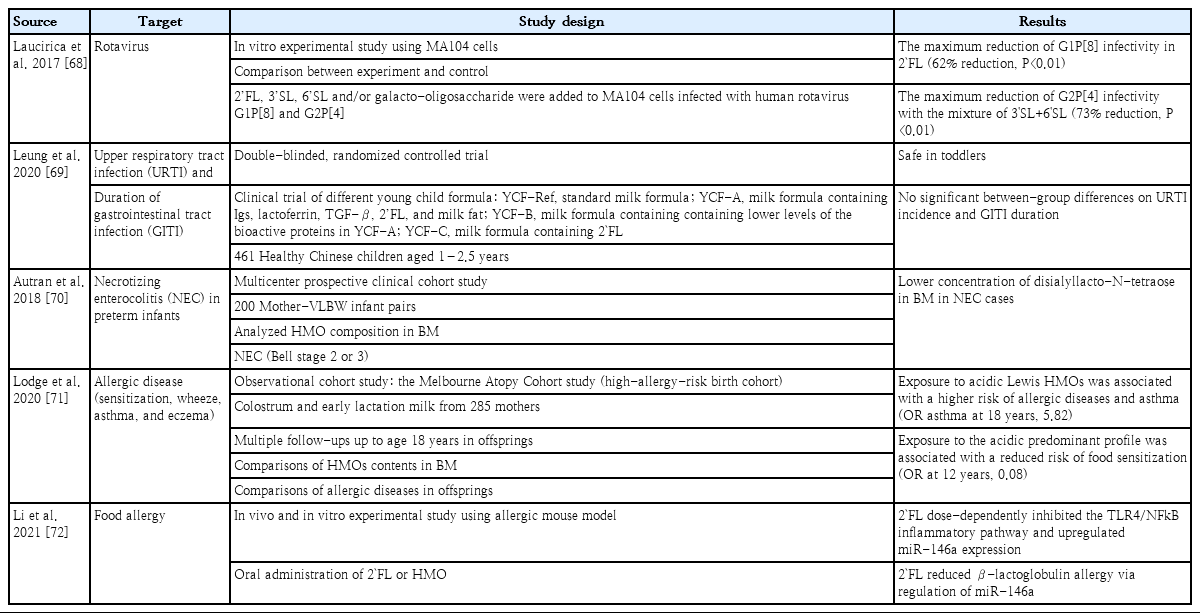
Human milk oligosaccharides (HMOs) is the third most abundant substance in human milk, followed by lactose and lipids. The profiles of HMOs are quite diverse, with more than 200 distinct structures that differ from those of other mammals [28-30]. The secretor and Lewis genes determine HMO diversity [12]. HMOs are not digested by gastric acid and act as prebiotics by stimulating the growth of intestinal microbiota [12]. The interaction of HMOs occurs mainly in Bifidobacterium infantis, Bifidobacterium bifidum, Bacteroides fragilis, and Bacteroides vulgatus [31,32]. Another commensal bacteria, Escherichia coli, benefits indirectly from HMOs using metabolites from B. fragilis when HMOs are degraded [33]. HMOs also provide antimicrobial activities by increasing the sensitivity of Group B Streptococcus, Staphylococcus aureus, or Acinetobacter baumannii to antibiotics [34,35]. They also provide antiviral activity against rotavirus, norovirus, influenza, and human immunodeficiency virus by interacting with immune cells and inhibiting viral invasion [36,37]. In addition, as an anti-pathogen, 2'-fucosyllactose (FL) of HMOs acts as a soluble decoy receptor for Campylobacter jejuni and reduces colonization [38,39]. Lacto-N-fucopentaose I and 2'-FL reduce enteropathogenic E. coli adhesion and reduce pathogenicity by binding to heat-labile enterotoxin type 1 [40]. HMOs improve gut barrier function by promoting epithelial cell maturation via short-chain fatty acids, microbiota metabolites of HMOs, and glycocalyx alterations [12,41]. They can also enhance the local and systemic immune system by inhibiting Toll-like receptors and interacting with dendritic cells, leading to T-cell proliferation [12,42-44]. HMOs as immunonutrients play a role in promoting healthy gut microbiota, protecting against bacterial and viral infections, improving gut barrier function, and optimizing immune function (Table 2). Therefore, HMOs play a potential role in preventing infection and allergic diseases (Table 3) [45].
6. Human milk microbiota
The initial formation of neonatal intestinal microbiota depends on maternal microbiota via adherence to intestinal cells and suppression of inflammation by the interaction with dendritic cell and the induction of T regulatory cell production [46]. The maternal microbiota is affected by diverse conditions, such as the prenatal use of antibiotics, health status, body mass index, and gestational age [47]. The complex human milk microbiota changes over time and varies among mothers. However, they generally include Bifidobacterium and Lactobacillus spp, Streptococcus, Staphylococcus, Bacteroides, and Enterobacter [46,47]. The human milk microbiota can contribute to establishing healthy intestinal bacteria in neonates and infants. Lactobacillus in the human milk induces Th1 cytokines and natural killer cells. They can adhere to intestinal cells, leading to the increased colonization of beneficial bacteria and the inhibition of pathogenic adhesion in the gut [48,49]. Bifidobacterium may suppress interleukin-8 in the presence of pathogenic Salmonella [50] and Bacteroides may suppress inflammation in the laminar propria by increasing FOXP3 T cells using surface polysaccharide A [51]. Although human milk contains diverse beneficial microbiota, intestinal microbiota compositions differ between infants fed expressed human milk and those directly breastfed. The intestinal microbiota of expressed breastmilk-fed infants show higher amounts of potential pathogens such as Enterobacteriaceae but lower amounts of Bifidobacterium [47,52]. Human milk plays crucial roles in preventing infections and influencing long-term health like obesity or allergic diseases via diverse immune mechanisms [45]. However, further research is required regarding the combined effect of bioactive supplementation in formula.
Micronutrients
1. Iron
Infants and children with iron deficiency can be susceptible to infections due to impaired T-cell function which is associated with decreased production of interleukin 2 [53]. Iron supplementation can reverse the T-cell dysfunction.
2. Zinc
Zn, the second most abundant metal in the human body following Fe, is a cofactor involved in DNA synthesis and repair. It is fundamental in protein synthesis as well as cell growth and differentiation. Zn deficiency occurs in acrodermatitis enteropathica, chronic malabsorption such as short bowel syndrome, or long-term insufficient supplementation of total parenteral nutrition [3]. Inadequate Zn intake increases diarrhea and pneumonia, especially in children younger than 5 years of age, and Zn supplementation can reduce the duration of diarrhea and respiratory illnesses [54]. Zn acts as a signaling molecule in the immune system [55]. Zn deficiency induces lymphopenia caused by decreased B cell differentiation in the bone marrow and interferon-γ production [56,57].
3. Vitamins
Vitamins act as antioxidants by suppressing reactive oxygen radicals and activating antioxidant enzymes [58]. Antioxidants can play important roles in the immune response via phagocytic function, cytokine and immunoglobulin production, and cell- mediated responses [3]. Oxidative stress or free radicals are involved in the development of diverse illnesses including chronic inflammatory diseases, aging, and cancers. Vitamin E is a membrane-bound antioxidant that can act as a free radical scavenger that prevents damage from lipid peroxidation. When vitamin E deficiency is caused by chronic cholestasis or a chronic malabsorptive state, decreased activities of antioxidative enzymes and increased hepatic lipid peroxidation can occur. Vitamin C, a hydrophilic antioxidant that cannot be synthesized by the human body, acts as a cofactor in collagen synthesis by activating hydroxylase by reducing Fe3+ to Fe2+. It is involved in inhibition of the redox potential under oxygen radical reactions in the body [59]. Excess free radial generation and oxidant cellular damage have been postulated in the pathogenesis of NEC in preterm infants and inflammatory bowel disease in children and adults. Although further clinical studies are necessary, vitamins can be used as antioxidant and immunologic nutrients [60,61].
Immunonutrition for preterm infants
Preterm infants are vulnerable nutritionally and immunologically because they are more susceptible to unstable clinical conditions than term infants. Therefore, immunonutrition is more important for preterm than term infants. Immunonutritional clinical strategies can focus on preventing inflammatory or infectious issues that might be encountered in a neonatal intensive care unit. These strategies include feeding human milk (due to its diverse immunologic bioactive substances), providing early introduction and fast advancement of enteral feeding, and minimizing iatrogenic harm [7,62,63]. Although further research is needed to determine the appropriate strain, effective dosage, clinical approval, and quality and safety issues, the probiotics, prebiotics, or synbiotics supplementation to prevent NEC or sepsis can be an immunonutritional strategy for premature infants [64,65].
Immunonutrition during the coronavirus disease 2019 pandemic
During the coronavirus disease 2019 (COVID-19) pandemic, the whole world is struggling to find treatment regimens and effective vaccines based on immunologic knowledge. Immunonutrition is emerged as an adjuvant dietary supplementation technique. Although severe acute respiratory syndrome coronavirus 2 is thought to be transmitted mainly via the respiratory route, gastrointestinal symptoms are often manifested at first in infants, suggesting that it can invade enterocytes [66]. Early human milk feeding can promote an intestinal environment of tight enterocyte junctions by diverse bioactive substances and can contribute to reinforcing innate defense in neonates and infants. Therefore, early breastfeeding may provide prevention during the viral pandemic [67]. Clinical trials using a combination of dietary supplements such as vitamin C, vitamin D, probiotics, and Zn are currently in progress [55]. The clinical and theoretical effects of nutrients on the immune system based on immunonutrition during this pandemic are very attractive. However, clinical and preclinical data are quite limited, and more well-designed studies are necessary.
Conclusion
Human milk is a major source of immunonutrients for healthy and sick babies. Bioactive substances in human milk can support neonatal and infantile immune systems directly and indirectly. HMOs are likely to have prebiotic effects that can enhance the colonization of beneficial intestinal bacteria. However, adding HMOs to standard or preterm formula requires more detailed future studies. Many micronutrients and vitamins may be supplemented as anti-inflammatory immunonutrients for infants. However, the toxicity of some nutrients from an overdose should be considered. Extensive studies of the effects of immunonutrients in the developing immune system have been performed (Tables 2, 3). Moreover, during this viral pandemic, the scientific interest in immunonutrition is increasing and further research is warranted to determine its clinical applications.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.