Diagnosis and management of asthma in infants and preschoolers
Article information
Abstract
Asthma is one of the most common chronic disease affecting children, and it often starts in infancy and preschool years. In previous birth cohorts, frequent wheezing in early life was associated with the development of asthma in later childhood and reduced lung function persisting into adulthood. Preschool wheezing is considered an umbrella term for distinctive diseases with different clinical features (phenotypes), each of which may be related to different underlying pathophysiologic mechanisms (endotypes). The classification of phenotypes of early wheezing is needed to identify children at high risk for developing asthma later who might benefit from early intervention. However, diagnosis of asthma in infants and preschoolers is particularly difficult because objective lung function tests cannot be performed and definitive biomarkers are lacking. Moreover, management of early asthma is challenging because of its different phenotypic presentations. Many prediction models and asthma guidelines have been developed to provide useful information for physicians to assess young children with recurrent wheezing and manage them appropriately. Many recent studies have investigated the application of personalized medicine for early asthma by identifying specific phenotypes and biomarkers. Further researches, including genetic and molecular studies, are needed to establish a clear definition of asthma and develop more targeted therapeutic approaches in this age group.
∙ Asthma in infants and preschoolers involves heterogeneous phenotypes.
∙ Asthma diagnosis is based on symptom patterns, therapeutic responses, and the presence of risk factors with careful consideration of differential diagnosis.
∙ Daily inhaled corticosteroid therapy remains the most effective strategy for managing persistent asthma symptoms irrespective of phenotype.
∙ Future research, including genetic and molecular studies, is needed to develop a clear definition of asthma and personalized therapeutic approaches.
Graphical abstract.API, asthma predictive index; EVW, episodic viral wheeze; ICS, inhaled corticosteroid; SABA, inhaled short-acting beta-2 agonist; LTRA, leukotriene receptor antagonist; Pref, preferred; Alter, alternative. a)Preferred in the children with positive API. b) Further studies are needed.
Introduction
Asthma is an inflammatory disease of the airways characterized by typical patterns of symptoms, such as recurrent episodes of wheezing, coughing, shortness of breath, and chest tightness. These features are associated with hyperresponsiveness of the asthmatic airways to inhaled irritants or endogenous stimuli that cause airway obstruction [1].
Asthma is one of the most common chronic disease affecting children, and it often starts in infancy and preschool years. Previous studies demonstrated that frequent wheezing in early life is associated with the development of asthma in later childhood and reduced lung function persisting into adulthood [2-4]. Early diagnosis of asthma is, therefore, important to avoid treatment delay and reduce morbidity. However, it is difficult to clearly define “asthma” in preschoolers because the underlying pathophysiology in this age group is poorly understood yet and there are no definitive biomarkers, and also because objective pulmonary function tests are unavailable [5].
Wheezing, a sign of airway obstruction, is very common in young children, who have much smaller airways than older children and adults. Their airways are so small that even a small amount of inflammation caused by viral infection can induce flow limitation and wheezing. In some children, wheezing is the first episode of asthma and often triggered by viral respiratory infection. Half of preschool children experience at least one episode of wheezing, and a third of them will have persistent wheezing and develop asthma at school age [6]. Thus, the classification of phenotypes of early wheezing is needed to identify the children at high risk of developing asthma later who might benefit from early intervention.
This article aimed to reach a consensus regarding the diagnosis and management of asthma in young children through a review of recent studies and current asthma guidelines.
Phenotypes of wheezing in infants and preschoolers
1. Longitudinal birth cohort studies
Wheezing is very common and heterogeneous in early life and shows different outcomes during childhood. Preschool wheezing is considered an umbrella term for distinctive diseases with different clinical features (phenotypes), each of which may be related to different underlying pathophysiologic mechanisms (endotypes) [7].
The Tucson Children’s Respiratory Study (TCRS), the first longitudinal birth cohort study to characterize the phenotypes of early wheezing, reported 3 phenotypes: (1) transient early wheeze (wheeze by age 3 years, but not age 6 years); (2) persistent early wheeze (wheeze starting before age 3 years and persisting at age 6 years); and (3) late-onset wheeze (wheeze starting after age 3 years and persists at age 6 years). Two phenotypes, persistent early and late-onset wheeze, were associated with atopy, bronchial hyperresponsiveness, reduced lung function by school age, and an increased risk of asthma in adolescence [6,8].
Another historic birth cohort was the Avon Longitudinal Study of Parents and Children (ALSPAC), which added 2 more phenotypes to those of the TCRS (i.e., transient early, persistent early, and late-onset wheeze): (1) prolonged early wheeze (onset at 6–54 months with peak prevalence at 30 months and remission after 69 months); and (2) intermediate-onset wheeze (onset at 18–42 months that persists) [9]. Intermediate-onset wheeze was characterized by the strongest association with atopy, lower lung function, and increased airway hyperresponsiveness. Transient and prolonged early wheezing were not associated with atopy, but were weakly associated with airway hyperresponsiveness and impaired lung function during school age. Persistent wheeze showed an intermediate association with these outcomes. In summary, the Avon study showed that a preschool wheeze that starts after 18 months of age and persists is most strongly associated with the later development of asthma, reduced lung function, and a high fractional exhaled nitric oxide in adolescence, which might show the timing of environmental influences on the initiation of atopic wheezing during early childhood [9]. Wheezing phenotypes and their characteristics identified in the ALSPAC study were confirmed by the Prevention and Incidence of Asthma and Mite Allergy (PIAMA) study [10].
The further characterization of wheezing phenotypes has been continued in recent birth cohorts, which have focused on environmental influences and the effect of early-life intervention. The Canadian Asthma Primary Prevention Study (CAPPS) included the high-risk infants whose parents had allergic diseases and classified them into 3 wheeze trajectory phenotypes: (1) early-transient, who did not develop asthma; (2) low-progressive, who gradually developed asthma; and (3) early-persistent, who showed a higher risk of asthma development [11]. Early-life interventions, including the encouragement of breastfeeding, supplementation of partially hydrolyzed formula, and avoidance of common allergens or environmental tobacco smoking (ETS) significantly decreased persistent wheezing only in the infants with early-persistent phenotype. This study also showed that wheezing in the second (but not first) year of life is a strong risk factor for asthma, a clinically important distinction that was not evident in classically defined wheeze phenotypes [11].
The Urban Environment and Childhood Asthma (URECA) study identified 5 phenotypes among high-risk, inner-city children. Asthma frequently developed in children with high wheeze/ low atopy and high wheeze/high atopy, infrequently developed in children with low wheeze/high atopy and low wheeze/low atopy, and was absent in children with transient wheeze/low atopy. This cohort study showed that early-life environmental exposure differentially associated with specific phenotypes, and particularly indicated that exposures to allergens, microbes, stress, and ETS might specifically modify the risk for high wheeze phenotypes with and without allergic sensitization [3].
2. Episodic viral wheeze and multiple trigger wheeze
In 2008, the European Respiratory Society (ERS) Task Force proposed the following clinical classification of preschool wheezing: (1) episodic viral wheeze (EVW); and (2) multiple trigger wheeze (MTW). EVW was defined as wheeze during discrete episodes of viral colds with no symptoms between episodes, and MTW was defined as wheeze not only during episodes of respiratory infection but also between episodes during activity, crying, or laughing [12]. The ERS Task Force introduced these wheezing phenotypes to guide treatment. However, several studies reported that the MTW or EVW phenotype switched over time in many preschool children with recurrent wheeze, demonstrating a limitation of this classification for decision-making in clinical care [13,14]. Moreover, the triggers of wheezing might not always be easy to identify, and the reliable distinction between EVW and MTW by history taking might be impossible in clinical practice [14].
EVW and MTW in preschool children did not match the longitudinally derived wheeze phenotypes of transient early wheeze and persistent wheeze (in the ALSPAC and PIAMA cohorts), respectively. However, this classification may predict who will or will not develop asthma at school age. One study reported that preschool children with stable MTW had a significantly increased risk of asthma [14], while another showed a high risk of asthma at 5–10 years of age among preschool children who had EVW severe enough to require hospitalization [15]. The authors recommended that severe EVW in preschool children should not be considered a transient disease but should be followed up and evaluated seriously.
In summary, longitudinal birth cohort studies suggest that more studies on genetic and environmental factors associated with different phenotypes of early wheeze are needed to elucidate the origin of childhood asthma and the effects of early intervention. Many studies on symptom-based classification of wheeze (EVW and MTW) indicate that frequency and severity of early wheeze should both be considered in making a treatment decision, irrespective of phenotype. In real-life clinical situations, the allocation of individual children to these phenotypes is challenging and the clinical usefulness of these phenotyping remains a subject of further investigation.
Bronchiolitis: the first wheezing episode of childhood asthma?
Bronchiolitis, a lower respiratory tract infection (LRTI), is the most common cause of hospitalization in infancy, and severe bronchiolitis is known to be associated with the later development of childhood asthma.
Bronchiolitis is generally considered a single disease entity, but many studies have reported that it is a heterogeneous condition [16,17]. There are increasing and convincing evidences showing that not all viral bronchiolitis corresponds to the same clinical condition, and that affected patients have high heterogeneity in clinical presentation, immune responses, and response to bronchodilators, and progression to recurrent wheezing or asthma [17]. The upper age limit for the diagnosis of bronchiolitis varies among countries; that is, bronchiolitis is defined in infants aged up to 24 months in North America and the United Kingdom, 12 months in some European countries, and 18 months in Australia [16]. The causative virus and the inclusion or exclusion of patients with a previous history of wheezing are additional sources of variability [16,17].
Most children with asthma that starts before 6 years of age initially present with bronchiolitis during infancy (the first episode), and asthma in infancy and preschoolers overlaps with virtually all traditional definitions of bronchiolitis [18]. Moreover, most exacerbations of preschool asthma are triggered by the same viruses implicated in bronchiolitis. Thus, we asked whether the wheezing infant has “bronchiolitis” (i.e., should receive supportive care) or “preschool asthma” (i.e., should receive early intervention with appropriate treatment) [18]. In fact, these 2 groups cannot readily be distinguished on a clinical basis and require clarification in further studies [17,18].
The relationship between respiratory syncytial virus (RSV) and asthma or atopy is known to be relatively low [19,20], but a recent study showed that certain RSV genotypes (ON1 and BA) preferentially cause bronchiolitis in infants with a possible genetic predisposition toward asthma and atopy [21]. Rhinovirus (RV) bronchiolitis is associated with a high risk of subsequent asthma at school age, especially in children with early sensitization to multiple allergens, higher immunoglobulin E (IgE) levels, higher eosinophil counts, and a history of wheezing [22,23].
A recent prospective cohort study of infants hospitalized for bronchiolitis identified 5 distinct clinically meaningful metabotypes by profiling the nasopharyngeal metabolome, and those with the metabotype characterized by high inflammatory amino acids and low polyunsaturated fatty acids were at the highest risk of developing asthma by 5 years of age [24].
Most current bronchiolitis guidelines define viral bronchiolitis as a single respiratory syndrome and recommend no active treatment options other than supportive care irrespective of viral etiology, host responses, or risk factors. However, recent studies suggest that it is reasonable to consider pharmacological treatment, that is, bronchodilators or corticosteroids, in infants with moderate to severe respiratory distress, particularly in those with risk factors for asthma [17]. Two separate randomized trials of systemic corticosteroid use in children with first-time RV bronchiolitis reported long-term efficacy in reducing the risk of asthma development [19].
In summary, although previous data indicated that bronchiolitis should be treated on a more personalized basis and that children with first wheezing episodes and risk factors for asthma may require early intervention, evidence remains insufficient. Further studies to define the phenotypes of viral bronchiolitis based on clinical and molecular levels and larger trials to support phenotype-based treatments are needed.
Prediction models for childhood asthma
Many birth cohort studies demonstrated that various wheezing phenotypes coexist during preschool years and approximately 30% of infants and preschoolers with recurrent wheezing develop asthma at school age [6]. Early identification of and intervention for children at risk of developing asthma may be crucial to improve the prognosis of the disease. Thus, during the last 2 decades, many asthma prediction models have been developed based on the risk factors associated with persistent wheezing identified in previous cohort studies.
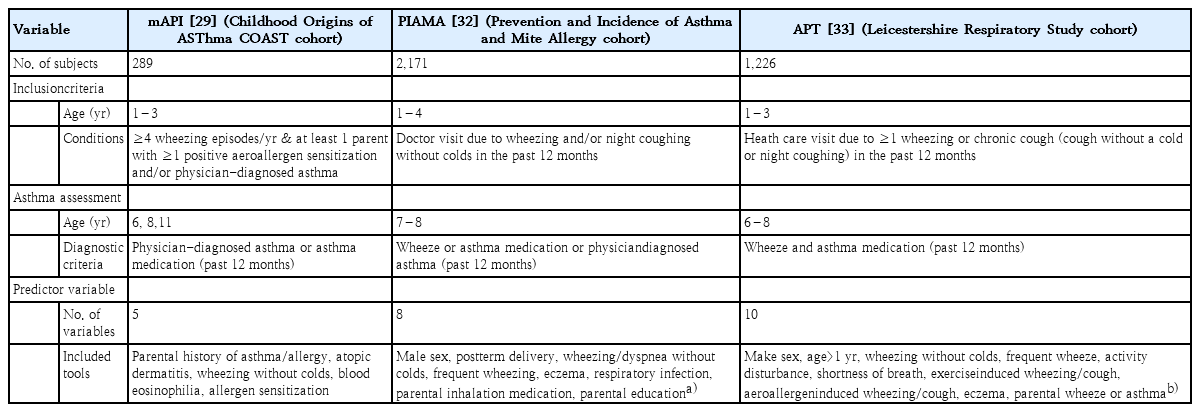
In 2000, the first model, the original asthma predictive index (API), originated from the TCRS and defined 2 prediction rules: loose API and stringent API [25]. The API, developed in the general population, has been the most popular prediction model and is commonly considered the reference to which newly developed models are compared. Children with a positive loose index were reportedly 4 times more likely than those with a negative loose index to have active asthma at school age (sensitivity, 42%; specificity, 85%; positive likelihood ratio [LR+], 2.8). Children with a positive stringent index were reportedly 7 times more likely to have active asthma at school age (sensitivity, 16%; specificity, 97%; LR+, 5.3) [25].
The API has been validated in other cohort studies and new populations, and the stringent API has been the most commonly tested model in different cross-sectional and case-control studies [26,27]. A recent population-based cross-sectional study in Korea of 916 preschool children showed a significant association between questionnaire-based current asthma and API, suggesting that the API can be used as a diagnostic tool for asthma with reasonable accuracy in preschool children [28]. The API was modified by replacing the clinical diagnosis of allergic rhinitis with evidence of allergic sensitization, a more objective criterion (Table 1). The modified API (mAPI) was developed in the high-risk COAST (Childhood Origins of ASThma) cohort of infants with at least 1 atopic parent (a parent with positive aeroallergen sensitization and/or physician-diagnosed asthma) [29]. A positive mAPI increased the probability of an asthma diagnosis later in school age to nearly 90% in preschoolers with atopic parents and frequent wheezing [29].
The API was mentioned in the Global Initiative for Asthma (GINA) guideline [1], and the mAPI was described as useful for identifying children who are more likely to respond to inhaled corticosteroid (ICS) therapy in the Expert Panel Report 3 guideline of the National Asthma Education Prevention Program (NAEPP) [30]. Both API and mAPI have been used as recruitment tools in many randomized clinical trials as well as to identify preschool wheezers with the “type 2 high” endotype [31]. The API, the first and the most frequently used model, had a relatively good positive LR and its major strength is its simplicity, which makes it easy to use. However, its negative LR is insufficient to rule out the development of school-age asthma [31].
Other prediction models that were validated externally are the model from the PIAMA birth cohort and the asthma prediction tool (APT) from the Leicestershire cohort, both of which were developed in high-risk groups [32,33]. The PIAMA model proposed a somewhat complicated asthma prediction score (range, 0–55) using 8 predictor variables in preschool children who presented with wheezing and/or night coughing without a cold in the previous 12 months and showed that children with high scores (≥30) had a 42% risk of developing asthma later in school age [32]. The APT model was developed in preschool children who visited doctors with respiratory symptoms: ≥1 wheezing or chronic cough (cough without a cold or night coughing) in the previous 12 months and used 10 predictor variables. APT classified the children into low risk (score ≤5), medium risk (score 6–9), and high risk (score ≥10) groups and showed 16%, 48%, and 79% risks of developing asthma later, respectively [33].
Recently. existing prediction models were reviewed for their predictive ability for school-age asthma and clinical applicability [29,34]. There are many differences among the models in study design, study size, target population, predictor variables, diagnostic criteria of school-age asthma, and statistical methodology (Table 2).
Most prediction models developed to date have shown moderate predictive performance and modest generalizability when validated externally, but they remain far from perfect in terms of widespread implementation in clinical practice [29,34].
Diagnosis of asthma in infants and preschoolers: a review of clinical guidelines
It is particularly difficult to establish a diagnosis of asthma in infants and preschoolers, the reasons of which include difficulties in performing pulmonary function tests at this age, a lack of evidence for underlying airway inflammation, and the fact that the disease may subside during the childhood. The diagnosis of asthma in young children is most often based on symptom patterns, presence of risk factors, and therapeutic responses.
Many international and national asthma guidelines, including 4 major guidelines: the NAEPP, the Practical Allergy (PRACTALL) Consensus Report by the European Academy of Asthma and Allergy, Evidence Based Approach by the European Respiratory Research (ERS) task force, and GINA guidelines, have been developed during the past 2 decades to increase physicians’ awareness of the disease, improve diagnosis and management, and promote international collaboration in asthma research [35,36]. Current asthma guidelines commonly describe that diagnosis of asthma in infants and preschoolers is difficult because they express different patterns of wheezing illnesses, but most guidelines implicitly accept that the diagnosis of asthma can be established without an age limit. However, the ERS guideline denies to use the term “asthma” in preschool children with wheezing episodes since they might disappear over time [12].
Diagnosis of asthma in this age group is based on physician’s interpretation of the following clinical findings: (1) symptoms and signs of airflow obstruction (frequent wheezing, chronic cough, shortness of breath); (2) improvement of these signs and symptoms with asthma therapy; and (3) no clinical suspicion of an alternative diagnosis.
1. Symptoms and signs suggestive of asthma and preschoolers
Frequent wheezing and chronic cough are the most common symptoms of asthma in young children. A wheeze or cough that occurs recurrently, occurs during sleep, or is triggered by activity, laughing, crying, or exposure to cold weather or tobacco smoke in the absence of an apparent respiratory infection is consistent with a diagnosis of asthma.
In young children, a large proportion of wheezing episodes is virally induced, and it may be difficult to determine whether a wheeze with a respiratory infection is truly an isolated event or represents a recurrent clinical presentation of asthma. Although most current guidelines do not clearly describe how to manage this topic, Canadian guidelines suggest that a diagnosis of asthma should be suspected in children under 5 years of age with recurrent asthma-like symptoms or exacerbations, even if triggered by a respiratory infection [37].
Cough due to asthma is generally nonproductive and usually accompanied by wheezing or breathing difficulties. However, a prolonged cough without cold symptoms in young children is reportedly associated with asthma in later childhood independent of wheezing. Shortness of breath that occurs recurrently during exercise increases the likelihood of an asthma diagnosis. The exertion of crying and laughing in infants and toddlers may be equivalent to that of exercising in older children [1].
The presence of atopic dermatitis, a family history of allergic disease, peripheral blood eosinophilia, or increased serum IgE level is highly supportive, but not diagnostic, of asthma. Complementary tests, including imaging studies, an allergy work-up, and asthma predictive rules, play a secondary role. Pulmonary function tests are not considered necessary at this age [36].
2. Therapeutic trial
In children with recurrent asthma-like symptoms and wheezing on presentation, direct observation of improvement with an inhaled bronchodilator (short-acting beta-2 agonist [SABA]) by a physician confirms the diagnosis.
In children with recurrent asthma-like symptoms but no wheezing on presentation, a trial of treatment for at least 2–3 months with regular low-dose ICS and as-needed SABA may provide some guidance for the diagnosis of asthma. Responses should be evaluated by controlling symptom frequency and severity. Marked clinical improvement during treatment and relapse upon treatment cessation support a diagnosis of asthma [36].
3. Differential diagnosis
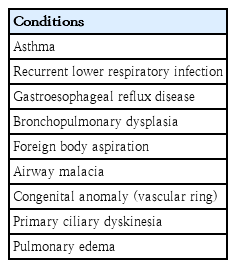
The differential diagnosis of wheezing in infants and preschoolers is complex and age-dependent. Symptoms that alert the physician to consider a diagnosis other than asthma include followings: wheezing which starts shortly after birth, continuous wheezing, failure to thrive, failure to respond to asthma medication, and no association with typical triggers, such as viral upper respiratory infection or exposure to specific allergens [1].
Several other diseases that might lead to wheezing in infants and preschool children are, in order of frequency, recurrent viral LRTI, gastroesophageal reflux disease, foreign body aspiration, airway malacia (tracheomalacia and/or bronchomalalcia), and extrinsic compression of the airways due to congenital anomalies (vascular ring). Children who were born prematurely with a low birth weight and treated with mechanical ventilation and prolonged oxygen supplementation frequently developed bronchopulmonary dysplasia and might have airway hyperreactivity and asthma symptoms later [38] (Table 3).
Management of asthma in infants and preschoolers
The effective management of early-life wheezing/asthma is very important because irreversible impairment of lung function may occur in infancy and preschool years. This age group tends to have significantly higher exacerbations caused by frequent respiratory infections than older children, and repeated and cumulative lung injuries might affect normal lung growth and asthma persistence [39]. A recent study showed the association between asthma control trajectories in preschoolers and disease remission: the worse the control, the lower the likelihood of remission [40].
1. Goals of treatment
The main goals of therapeutic interventions for early asthma are to control symptoms (cough, wheezing, and breathlessness) and prevent acute exacerbations. Because asthma clearly originates during the preschool years, the final goal of early intervention in high-risk children might be disease modification (i.e., prevention of subsequent asthma). However, no currently known treatments modify the natural history of the disease [1].
Current asthma guidelines have proven useful in the standardization of treatment and reduction of adverse effects. However, previous studies that included early wheezers with heterogeneous phenotypes within the same clinical trials resulted in negative findings for the whole population but positive findings for specific phenotypes [5]. Many recent studies investigated the concept of “personalized treatment” by identifying phenotypes and biomarkers in preschoolers with asthma.
2. Phenotype-directed management of asthma in infants and preschoolers
1) Intermittent asthma
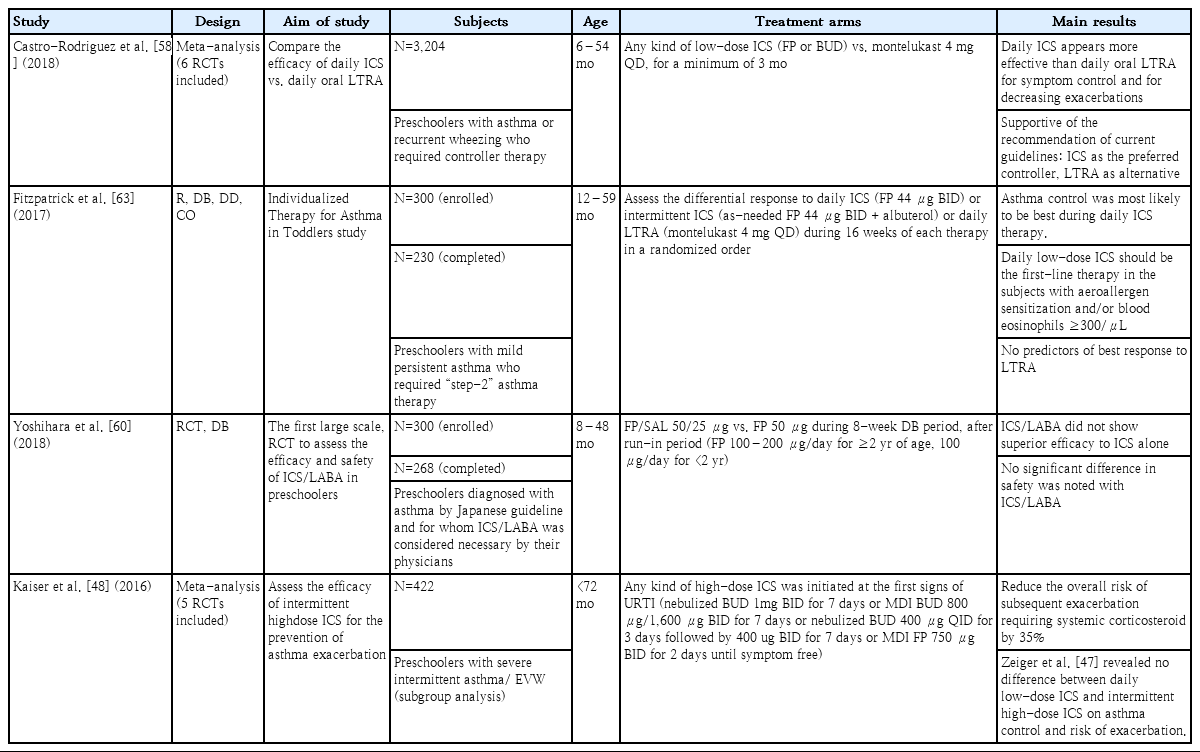
Asthma guidelines recommend that preschoolers (5 years and younger) with mild intermittent symptoms be treated with asneeded inhaled SABA (step 1). However, children with severe intermittent asthma or EVW who experience severe wheezing during acute respiratory viral illnesses but usually remain asymptomatic between episodes, present a different phenotype [12,41]. Two treatment strategies have been suggested for these children: daily therapy to prevent episodes and short-term therapy during episodes.
Previous studies demonstrated that daily low-dose ICS effectively reduced the frequency and severity of wheezing exacerbations versus placebo in preschoolers with asthma risk factors (positive mAPI) [42,43]. In the PREvention of Virally Induced Asthma (PREVIA) study, daily leukotriene receptor antagonist (LTRA) therapy significantly reduced the rate of wheezing exacerbations in young children (2–5 years of age) with intermittent asthma,44) but a later meta-analysis of 5 studies showed no evidence of clinical benefit [45].
Intermittent therapy with high-dose ICS or LTRA only during acute respiratory illnesses has been attempted in preschoolers (1–5 years of age). The Acute Intermittent Management Strategies (AIMS) trial, a randomized, double-blind, placebo-controlled study, demonstrated that intermittent high-dose ICS significantly reduced acute exacerbations over the 12-month period, particularly in the children with positive mAPI [46]. The Maintenance and Intermittent ICS in Wheezing Toddlers (MIST) trial was conducted in the children with positive mAPI and showed similar effects in reducing the rate of acute exacerbations compared with daily low-dose ICS [47]. A metaanalysis also demonstrated that intermittent high-dose ICS started at the onset of a respiratory infection reduced severe exacerbations versus placebo [48]. No significant difference was observed in terms of a reduction of exacerbations and adverse effects between daily low-dose ICS and intermittent high-dose ICS, but data remain insufficient [49].
Episodic use of LTRA has also been attempted in children with EVW. A previous study reported that children treated with montelukast for at least 7 days starting at the onset of a respiratory infection experienced a 28.5% reduction in exacerbations, and this result was more evident in preschoolers (2–5 years of age) [50]. In another report, however, no significant difference was observed in the reduction of acute episodes between the montelukast and placebo groups [51]. Despite these controversial results, both studies showed that montelukast significantly reduced symptom severity during episodes versus placebo [50,51]. The ERS Task Force report recommends the episodic use of montelukast for the treatment of EVW [12].
2) Episodic macrolide therapy for severe intermittent asthma
Episodic azithromycin therapy was recently introduced as a treatment option for children who present with severe intermittent LRTI-associated asthma [52]. Allergic, eosinophilic, steroid-responsive asthma in preschool children is relatively well identified, but the nonallergic group that constitutes the majority of preschool asthma cases has not been studied much yet [3].
Although respiratory viruses are frequently associated with recurrent wheezing in preschool children, bacteria such as Streptococcus pneumoniae, Moraxella catarrhalis, and Hemophilus influenzae often affect the risk of wheezing [53]. The role of macrolide antibiotics has been examined for the treatment of acute episodes associated with bacterial infection. The Azithromycin for Preventing the Development of Upper Respiratory Tract Illness into Lower Respiratory Tract Illnesses (APRIL) [54] and the Copenhagen Prospective Studies on Asthma in Childhood cohort (COPSAC) [55] trials were conducted in the preschool children who had recurrent episodic wheezing associated with LRTI severe enough to require systemic corticosteroids and/or emergency department visits, but were not treated with daily controller medicine. These 2 studies showed that intermittent azithromycin administered early in LRTI episodes significantly decreased disease severity, and this response was independent of the children’s mAPI status and detected viruses. Their results suggest that the early use of azithromycin can be a treatment option in nonatopic children with severe intermittent asthmalike symptoms, whereas intermittent high-dose ICS is preferred for those with positive mAPI and allergic sensitization [54-56]. However, another study reported no difference in the severity of the acute episodes between the azithromycin-treated group and the placebo group, although the characteristics of the enrolled subjects differed somewhat from those of previous trials [57].
In summary, the exact role of episodic azithromycin therapy in young children with recurrent wheezing remains uncertain, and another major concern is increasing macrolide resistance. Further studies are needed to investigate the predictors or biomarkers that can identify the subgroup of children who might benefit most from this treatment option.
3) Persistent asthma
Current asthma guidelines suggest regular controller therapy for children with persistent symptoms. The children who have ≥3 or 4 episodes of wheezing lasting ≥24 hours and affecting sleep in the previous year, ≥1 or 2 times symptoms per week for a period of more than 4 weeks, or ≥2 exacerbations requiring oral corticosteroids within 6 months should be managed with daily controller therapy [1]. Most guidelines suggest a stepwise approach to control persistent asthma symptoms (steps 2–4), and only the NAEPP guidelines suggest additional steps 5 and 6 [30]. (Table 4) All guidelines recommend daily ICS as the preferred controller and LTRA as an alternative or add-on therapy, as supported by a recent systematic review [58]. Although neuropsychiatric events have been reported infrequently in patients taking montelukast, preschool children do not appear more vulnerable to such events than older children or adolescents [59].
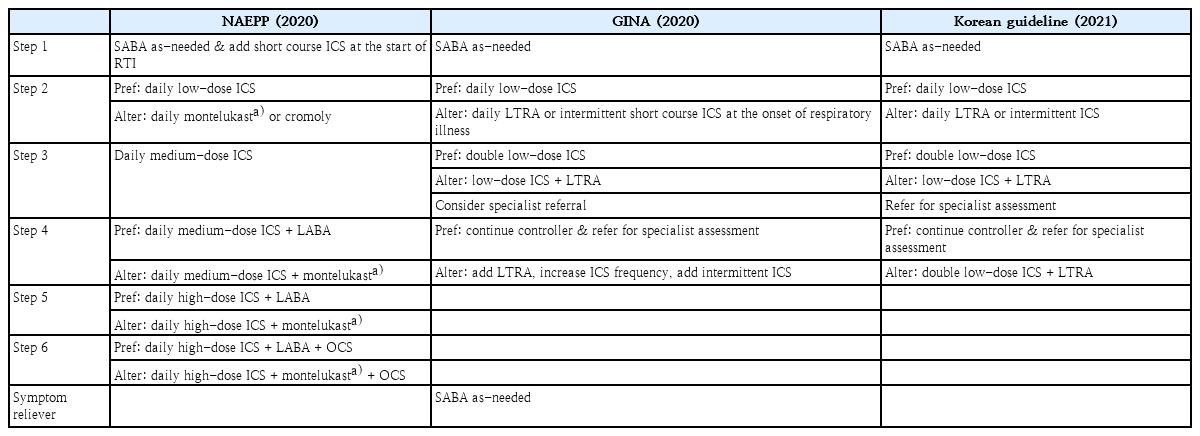
Summary of stepwise approaches for management of asthma in preschoolers suggested by current guidelines
Combination of ICS and long-acting beta-2 agonist (ICS/LABA) is widely used as maintenance therapy for both children and adults. However, this combination therapy has not yet been adequately studied for preschool asthma, and therefore, it is not officially recommended by most age-specific guidelines. Only the NAEPP guidelines recommend ICS/LABA therapy for young children who are not controlled with medium-dose ICS based on the studies in older children [30]. (Table 4) Two recent studies, including the first randomized controlled trial (RCT) [60], have reported the efficacy and safety of ICS/LABA therapy in young children, demonstrating no superior efficacy to ICS alone and no clinically significant difference in safety [60,61]. On the other hand, another retrospective study reported that ICS/LABA therapy was highly effective and well-tolerated in preschoolers with moderate to severe asthma [62]. Properly designed clinical trials are needed to consider the incorporation of ICS/LABA therapy in the current asthma guidelines for this age group.
Asthma in young children has different phenotypic presentations that may result in different responses to controller medication. The GINA guidelines do not yet suggest phenotypespecific treatment options [1]. However, the NAEPP guidelines proposed that preschool children with positive mAPI are more likely to respond to ICS therapy [30]. The concept of personalized medicine for the management of preschoolers with recurrent wheezing/asthma has recently been suggested. The Individualized Therapy for Asthma in Toddlers (INFANT) trial was the first to examine the relationship between the phenotypic features and biomarkers of asthma and the response to asthma medication [63]. The INFANT study included young children aged 12–59 months who were candidates for step 2 therapy and assessed the differential response to 3 treatment options: daily ICS, as-needed ICS, and daily LTRA. All participants were treated with each therapy for 16 weeks in a randomized order. The results showed that 74% of the children demonstrated clinically relevant improvements in response to one treatment versus the others, most often daily ICS [63]. The best response to daily ICS was observed in children with aeroallergen sensitization and a blood eosinophil count ≥300/μL, the evidences of type 2 inflammation. In addition, high serum eosinophil cationic protein levels ≥10 μg/L and pet sensitization predicted a better response to daily ICS, but no predictors were noted for those who responded better to daily LTRA or asneeded ICS.
3. Daily ICS safety in infants and preschoolers
Among the adverse effects associated with daily ICS therapy, growth suppression is the most concerning for physicians and parents. Although many studies of prepubertal school-aged children demonstrated a clinically modest but statistically significant effect on linear growth, these findings should not be directly extrapolated to young children, whose linear growth is influenced by factors other than growth hormone (e.g., nutrition) [5]. The few RCTs on preschoolers to date reported that the growth-suppressive effects of ICS therapy are generally small and appear to improve over time in most children [5]. However, data on the long-term effects of daily moderate- to high-dose ICS therapy are lacking and individual susceptibility to ICS may be variable. Consequently, it is prudent to monitor linear growth in all children treated with daily ICS and aim to use the lowest effective dose to maintain asthma control [49].
In summary, irrespective of the apparent phenotype, daily ICS therapy remains the most effective strategy for infants and preschoolers with persistent asthma. Many recent studies have investigated the concept of personalized treatment and suggested that daily low-dose ICS should be the initial therapy for young children with aeroallergen sensitization and/or peripheral blood eosinophilia. Recent studies on the management of asthma in preschoolers are summarized in Table 5.
Conclusion
Diagnosis of asthma in infants and preschoolers is particularly difficult because objective lung function tests cannot be performed and definitive biomarkers are lacking. Moreover, management of asthma is challenging due to different phenotypic presentation of early asthma. Many recent studies have investigated the application of personalized medicine for early asthma by identifying specific phenotypes. Further researches, including genetic and molecular studies, are needed to establish a clear definition of asthma and develop more targeted therapeutic approaches in this age group.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.