Research trends on causes of Kawasaki disease in the COVID-19 era: focus on viral infections
Article information
Abstract
Despite studies on the etiology of Kawasaki disease (KD) ongoing for half a century since its discovery, its cause has not yet been clearly identified. Although the clinical, epidemiological, and pathophysiological characteristics of KD are presumed to be closely related to infectious diseases, studies of various pathogens to identify its etiology have been actively conducted. To date, bacteria, fungi, and viruses have been investigated to determine the relationship between KD and infection, among which viruses have attracted the most attention. In particular, during the coronavirus disease 2019 pandemic, there were many reports in Europe of a sharp increase in cases of Kawasaki-like disease (KLD), while conflicting reports that the prevalence of KD decreased due to thorough “social distancing” or “wearing mask” in Asian countries drew more attention regarding the association between KD and viral infection. Therefore, the differential diagnosis of KD from KLD with these similar spectra has become a very important issue; simultaneously, research to solve questions about the association between KD and viral infections, including sudden acute respiratory syndrome coronavirus 2, is drawing attention again. Moreover, a new concept has emerged that immune responses occurring in patients with KD can be caused by the pathogen itself as well as host cells damaged by infection. This paper summarizes the research trends into KD etiology and related pathophysiology, especially its association with viral infections, and present future research tasks to increase our understanding of KD.
Key message
∙ The etiology of Kawasaki disease (KD) is unclear, but its clinical, epidemiological, and pathophysiological characteristics are strongly associated with infectious diseases.
∙ In the coronavirus disease 2019 pandemic era, viruses are attracting the most attention. Sudden acute respiratory syndrome coronavirus 2 infection causes various hyperinflammation in children that require differentiation from KD.
∙ Immune responses in patients with KD may be induced by host cell damage. To effectively prevent and treat KD, the genetic background and immune responses of KD patients and triggering pathogens require identification.
Graphical abstract.Summary of paradigm for pathophysiological research in Kawasaki disease. KD, Kawasaki disease; COVID-19, coronavirus disease 2019; DDx., differential diagnosis; MIS-C, multisystem inflammatory syndrome in children.
Introduction
Although the coronavirus disease 2019 (COVID-19) pandemic has hampered daily life, it has shed new light on many aspects. The emergence of new diseases always intimidates people but occasionally aids the development of a new approach to the study of diseases that have remained unsolved.
Kawasaki disease (KD) is an acute systemic vasculitis that mainly manifests in children aged <5 years whose diagnosis usually depends on its clinical manifestations. KD is related to the patient's genetic sensitivity and risk of infection [1], and although various studies have examined its etiology for more than half a century since its introduction by Tomisaku Kawasaki in 1967 [2], it has yet to be clarified.
Kawasaki-like disease (KLD), also known as multisystem inflammatory syndrome in children (MIS-C), was reported for the first time in Europe last year [3] in pediatric patients with COVID-19. Although MIS-C shares some clinical features with KD, the 2 conditions are distinct. However, the reemergence of MIC-C reminded us of the apparent but unconfirmed association between KD and COVID-19 infection and raised expectations about KD pathology finally being resolved [4-6].
Recent reports [7-9] showed that the incidence of KD significantly decreased versus that in the same period during the COVID-19 pandemic, when mask wearing and social distancing were implemented in Asian countries, where the KD prevalence was high. In contrast, the number of KLD cases has significantly increased in the United States [10], Italy [11], and the United Kingdom [12], where COVID-19 was more prevalent. These conflicting phenomena raised the expectation that the association between KD and viral infections could be confirmed. Therefore, this study briefly summarizes the trends and results of research on the possible association between KD and infectious diseases, especially viral infections.
Research on cause of KD
In previous studies, KD was not controversial because abnormal immune responses in individuals with certain genetic backgrounds after infection are important mechanisms [1]. Although KD is considered highly associated with infectious diseases in clinical, epidemiological, and pathophysiological aspects, its pathology is among the most notable research topics.
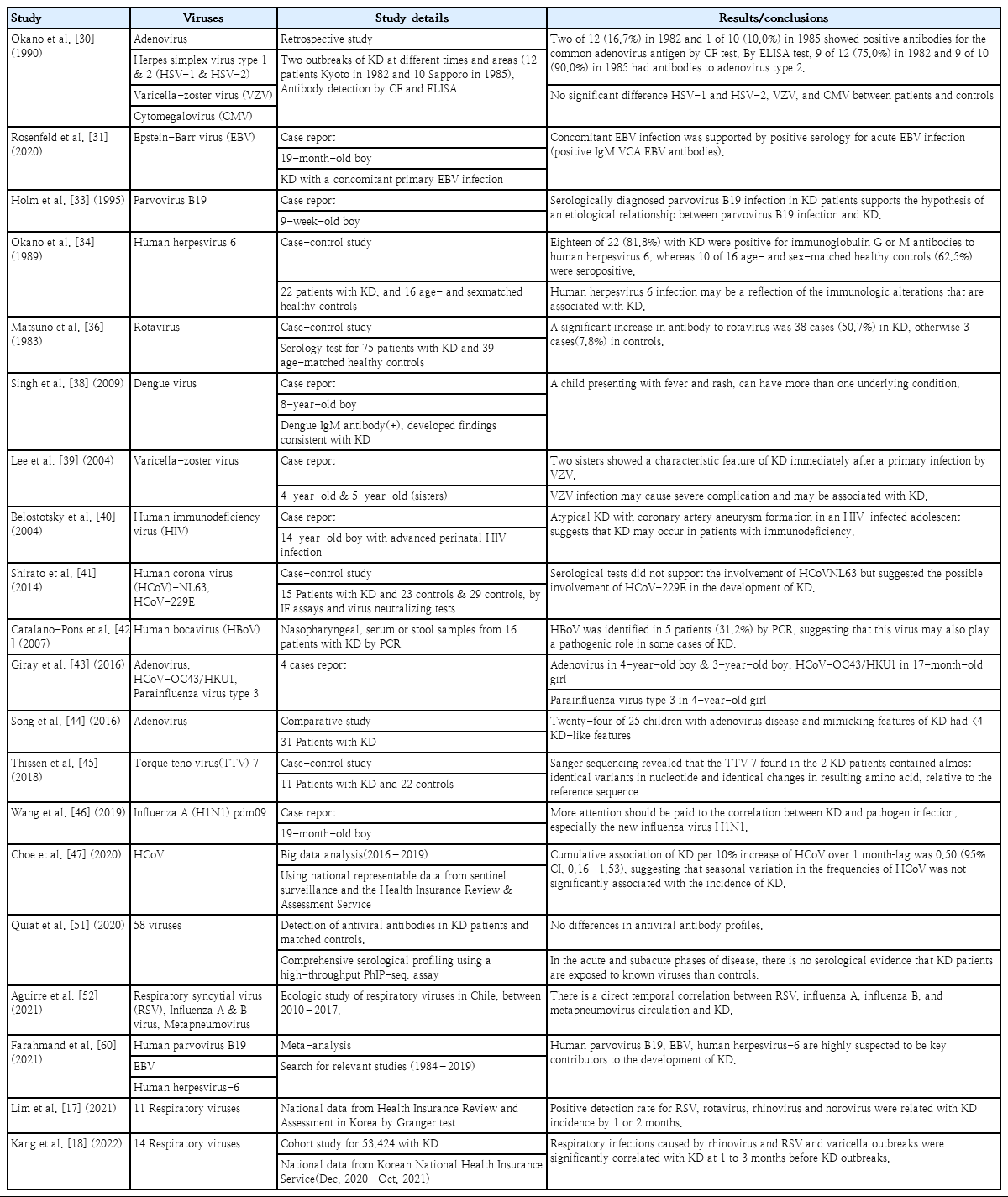
Table 1 summarizes the evidence suggesting a connection between KD and infection. First, most clinical symptoms included in the diagnostic criteria for KD (such as acute fever, skin rash, conjunctival injection, redness of the oral mucosa including strawberry tongue, and lymphadenitis) as well as accompanying respiratory symptoms are similar to those of acute infectious diseases. Epidemiologically, it is common in young children aged <5 years who are vulnerable to infections, and considering its occurrence in siblings of affected children [13,14], the high prevalence rate in countries in Northeast Asia [15], and the marked seasonal variations [16], it is believed to be an infectious disease characterized by epidemics. Recently, studies using big data and time series analysis of disease statistics have reported that various viral infection outbreaks precede KD [17,18]. Moreover, pathophysiologically, the pattern of explosive systemic vasculitis in the acute phase is associated with microbial toxins or superantigens, and the fact that systemic vasculitis is sometimes self-limiting is similar to viral infectious diseases. Immunologically, the initial neutrophilic predominance in peripheral blood and arterial tissues in KD is compatible with an innate immune response to acute infection [19].
Research on KD-associated infectious pathogens
Although the exact cause of KD remains unknown, studies are underway. To identify the pathogen that causes a certain disease, some conditions must be met. According to Nagata [1], if an infectious pathogen has the potential to induce KD, it should (1) frequently be detected in KD patients, (2) be associated not only with clinical symptoms of KD disease, (3) have a coronary artery lesion induction mechanism, and (4) have an epidemiological association.
Nagata [1] recently categorized 4 main theories about KD etiology: (1) direct infectious vasculitis, (2) autoantigen, (3) superantigen, and (4) RNA virus. However, none of these studies have been able to justify this epidemic.
To date, a wide variety of bacteria [20-29] and viruses [30-50] as well as the occasional fungus [51,52] have been reported as KD-associated infectious pathogens (Table 2). Studies of most KD-associated pathogens are based on the theory that these pathogens act as antigens or even superantigens in genetically sensitive individuals that result in inappropriate immune responses rather than directly infecting individuals.
First, Staphylococcus and Streptococcus species are at the center of the superantigen theory, which has attracted attention related to KD pathogenesis. Leung et al. [20] confirmed that the selective expansion of Vβ2+ T cells in most KD patients may be caused by Staphylococcus and Streptococcus species. Matsubara and Fukaya [22] provided the following evidence of Staphylococcus and Streptococcus superantigens in the KD pathogenesis: the skewed distribution of the Vβ repertoire, superantigen-producing bacteria isolated from KD patients, serological responses to superantigens produced by Staphylococcus aureus, and Group A Streptococcus from case-control studies. Animal models have demonstrated the hallmarks of a superantigen-mediated response. In addition, Yersinia pseudotuberculosis [24], Pseudomonas aeruginosa[ 26], Chlamydia pneumoniae [27], and Mycoplasma pneumoniae [28] are suspected causative bacteria, but this remains unconfirmed due to other opinions (Table 3).
Wang et al. [49] summarized that various common pathogens, mainly bacterial toxins and viral antigens, act as external superantigens in susceptible hosts, causing several diseases such as KD, toxic shock syndrome, and rheumatoid arthritis. In a study of previous or concurrent infections in patients with KD, Fernández-Cooke et al. [50] reported that 17.2% of patients had recent previous infections within the last 4 weeks, while 16.3% had microbiologically confirmed infections in the acute phase. They also emphasized that a standard protocol for microbial testing should be prepared to identify actual infections in patients with KD.
Examples of studies that estimate fungi as the cause of KD include the development of KD-like animal models by Takahashi et al. [53] that induced vasculitis in mice with Candida albicans extracts and that by Rodó et al. [54] that studied the relationship between KD and Candida species. Rodó et al. [54] explained the seasonal cluster phenomenon of KD as the possibility of aerosol transmission by Candida species via tropospheric winds.
These numerous reports imply that various pathogens are a potential cause of KD. Nevertheless, conclusive evidence that infections by these individual pathogens cause KD is lacking, and the possibility of concurrent infections has not been completely excluded [53].
Studies of association of KD and viral infection
In the theory of KD caused by infectious pathogens, bacteria were mainly mentioned first, but interest has gradually shifted toward viruses. The association between viruses and KD has been steadily attracting attention [30-51,55-60]. Speculation that a viral infection could be a KD etiology was triggered by the occurrence of seasonal outbreaks reported in Japan in 1979, 1982, and 1986 [61].
In the case of KD accompanied by viral infection, the virus is suspected to be the etiology as follows: Epstein-Barr virus (EBV) [31,32], parvovirus [33], dengue virus [38], varicella-zoster virus [39], human immunodeficiency virus [40], human bocavirus [42], human adenovirus [43], human coronavirus OC43/HKU1 [43], parainfluenza virus type 3 [43], torque teno virus 7 [44], and influenza A [45]. These reports are cases in which an infection with a related pathogen was confirmed in various samples extracted from patients treated for KD, but the possibility of accidental coinfections cannot be ruled out regardless of KD status. In case-control studies of viruses as the etiology of KD, adenovirus, human herpesvirus 6, rotavirus, and human coronavirus (HCoV) were reported. Okano et al. [30] reported that patients with KD with 2 outbreaks in 1982 and 1985 had a significantly higher positive rate of adenovirus antibodies than the control group. Meanwhile, Song et al. [44] emphasized that if adenovirus is detected incidentally in KD, it is necessary to carefully distinguish it from acute adenovirus infection itself. This is because human adenovirus is accompanied by long-term fever, skin rash, and increased inflammatory markers in children that can cause clinical characteristics very similar to those of KD. Okano et al. [34] reported that the human herpesvirus 6 antibody positivity rate was higher in 22 patients with KD than in 16 age-and sex-matched healthy controls. Matsuno et al. [36] reported that the positive rate of rotavirus antibodies was significantly higher in 75 KD patients and 39 age-matched controls. Shirato et al. [41] reported that HCoV-229E was more likely involved in the development of KD than HCoV-NL63 using immunofluorescence assays and virus neutralization tests. However, Choe et al. [47] analyzed nationally representative data from 2016–2019 in Korea and reported that seasonal variations in the frequencies of HCoV were not significantly associated with the incidence of KD. Quiat et al. [51] reported no serological evidence that KD patients were exposed to 58 known viruses compared to controls in the acute and subacute stages of KD.
Epidemiologically, although some differences exist among countries, Uehara and Belay [57] reported that KD occurs in clusters and community-wide outbreaks with distinct seasonality, indicating that KD can be caused by infectious agents such as viruses or agents that remain elusive. Similar results were reported of epidemiological investigations of big data by Korean researchers [17,18], suggesting that respiratory viruses may be causative pathogens that trigger KD among viral infections. Lim et al. [17] reported that several viruses, including human respiratory syncytial virus, rotavirus, norovirus, and human rhinovirus, precede KD by 1–2 months in the same cycle as the trend of KD diagnosis in a study of big data in Korea, supporting the possibility that the virus is the major cause of KD. Kang et al. [18] reported a cohort study of a time series analysis of children and youth in South Korea with KD, stating that respiratory infections caused by rhinovirus and respiratory syncytial virus as well as varicella outbreaks were significantly correlated with KD at 1–3 months before KD outbreaks. Aguirre et al. [52] reported a direct temporal correlation between respiratory syncytial viruses, influenza A, influenza B, metapneumovirus circulation, and KD through an ecological study of respiratory viruses in Chile in 2010–2017. Table 4 summarizes the major studies of viruses as KD-associated pathogens.
Rowley et al. [58,59] reported that CD8 T cells, oligoclonal A, and the upregulation of cytotoxic T cell and interferon pathway genes in the coronaries in fatal KD, or identifying cytoplasmic inclusion bodies in ciliated bronchial epithelium, supports a viral etiology, especially of RNA-associated viruses. However, since this requires a highly invasive tissue biopsy in KD patients and advanced molecular techniques, easier and safer tissue sampling methods should be performed.
Farahmand et al. [60] recently attempted the first systematic review and meta-analysis to investigate the association between different viral infections and KD development. Although some limitations exist in that their research data for acute KD and most studies were conducted in Japan, they found that human parvovirus B19 viremia (odds ratio [OR], 41.05; 95% confidence interval [CI], 5.13–328.28; I2=0%), EBV immunoglobulin M seropositivity (OR, 7.18; 95% CI, 3.65–14.12; I2=0%), and human herpesvirus 6 immunoglobulin G seropositivity (OR, 5.83; 95% CI, 1.06–32.01) were highly suggested as key contributors to the development of KD in children. They also hypothesized that KD is not caused by a single viral pathogen but is likely due to multiple viral pathogen infections.
Meanwhile, the relationship between KD and vaccination has also been reported for hepatitis A, hepatitis B, influenza, and rotavirus vaccines[ 62-67]. Miron et al. [62] reported KD cases that occurred one day after the second dose of hepatitis B vaccination, and Yin et al. [63] reported that KD occurred after the second dose of rotavirus vaccine and the first dose of hepatitis A vaccine at the same time. Shimada et al. [64] raised the possibility of vasculitis due to an autoimmune reaction to an influenza vaccine through a KD case that occurred after receipt of the second dose of influenza vaccine. In particular, Bonetto et al. [65] reviewed 75 studies of various vaccines and vasculitis over 20 years from 1994 to 2014 and reported that the influenza vaccine is more frequently associated with vasculitis than any other vaccine. However, Hua et al. [66] reported no evidence that vaccines increased the risk of KD in 107 cases of KD and 23 U.S. Food and Drug Administration–licensed vaccines across the United States in 1990–2007. Abrams et al. [67] reported that childhood vaccination was related to a decreased incidence of KD by analyzing data from the Vaccine Safety Datalink of the US in 1996–2006. Most recently, Peralta-Amaro et al. [68] reported a case of atypical KD in adults after COVID-19 vaccination (Table 5).
As mentioned above, epidemiological and pathological studies have proposed viruses as the most attractive etiology of KD, but consistent evidence is lacking that any particular virus is the etiology, so research on this topic should continue.
COVID-19 and KD
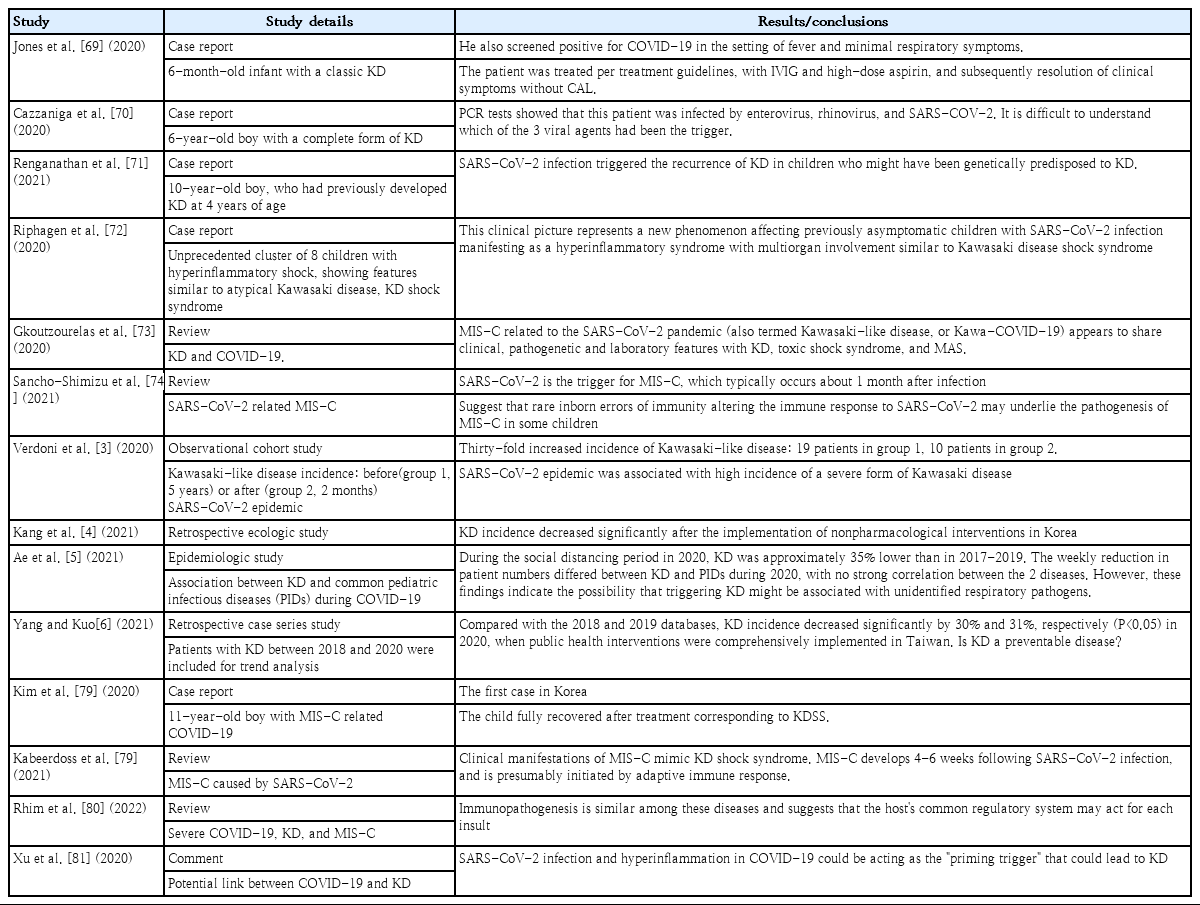
Verdoni et al. [3] initially reported that 10 cases of KLD occurred in just 2 months in Bergamo, Italy, the city hardest hit by COVID-19, increasing by 30-fold compared to 19 cases in the immediately preceding 5 years. Since then, many countries in Europe reported a series of positive responses to SARS-CoV-2 infection in patients with KLD [69-72], raising questions about whether there is a connection between KD and COVID-19. Jones et al. [69] reported a positive case of COVID-19 in 6-monthold infants with classic KD, and Cazzaniga et al. [70] reported a positive case in a 6-year-old boy. Renganathan et al. [71] reported an interesting case of KD recurrence after COVID-19 infection in a 10-year-old boy with a history of KD 4 years prior. Riphagen et al. [72] reported an unprecedented cluster of 8 children with hyperinflammatory shock, showing features similar to atypical KD, KD shock syndrome (KDSS), or toxic shock syndrome (TSS), during a 10-day period in mid-April 2020, 4 of whom had a family history of COVID-19.
On the other hand, in Asia, the KD prevalence decreased during the same period, raising the same issue of the connection between KD and viral infections, which was assumed to be the effect of thorough social distancing or mask wearing [4-6]. Kang et al. [4] reported a reduction in KD after nonpharmaceutical intervention through an ecological study. Ae et al. [5] reported that the incidence of KD in 2020 decreased by 35% versus the previous 3 years (2017–2019) in Japan; in Taiwan, Yang and Kuo [6] reported that, in 2020, Taiwan decreased by 30% and 31% compared to 2018 and 2019, respectively. However, the fact that the number of patients with KD or KLD varies widely among regions before and after the COVID-19 pandemic can be a result of differences in regional policies on infectious diseases affecting healthcare access. In other words, the decrease in KD prevalence in Asia may have been an underestimation caused by the COVID-19 pandemic, which reduced access to healthcare. Therefore, further studies are required in the future.
The curiosity about the association between COVID-19 and KD began with reports of clinical features similar to KD, although it sometimes showed a critical course [73,74]. This is more often reported in areas where COVID-19 is highly prevalent [75], but the new syndrome differs from actual KD; therefore, the World Health Organization and the Centers for Disease Control and Prevention of the US termed it MIS-C [76]. MIS-C is more common in older age groups than KD; the most frequent clinical features are gastrointestinal or neurological symptoms, and shock is also common, making it difficult to consider it the same disease [48,77-81]. Although there are some overlapping clinical manifestations of COVID-19 and KD, there are also clear distinctions. In South Korea, Kim et al. [78] reported the first case of MIS-C related to COVID-19 in an 11-year-old boy with clinical features of incomplete KD or KDSS in 2020.
Based on the data published thus far, MIS-C, severe COVID-19 infection without MIS-C, TSS, and KD are compared in Table 6. Notably, Rhim et al. [80] recently introduced the hypothesis that similar diseases associated with infection are based on a common immunopathogenesis, the “protein-homeostasis-system,” although clinical manifestations appear in various forms. In particular, this hypothesis is a new concept of the immune response that occurs in an individual, not only by the pathogen itself but by host cells damaged by infection, and is expected to bring new implications for the ongoing study of KD etiology.

Comparison of multisystem inflammatory syndrome in children (MIS-C), severe COVID-19 disease without MIS-C, toxic shock syndrome (TSS), and Kawasaki disease (KD)
However, Xu et al. [81] hypothesized that MIS-C due to SARS-CoV-2 infection acts as a “priming trigger,” leading to KD, as strong systemic inflammatory reactions may trigger coronary lesions. Xu et al. [81] also estimated that the absence of reported KD or KD-like symptoms observed in pediatric patients in China since the COVID-19 outbreak was due to differences in racial backgrounds and genetic susceptibilities. Unlike in Europe, in Korea, Japan, and Taiwan, the prevalence of KD decreased during the COVID-19 pandemic [4-6]. This phenomenon is attributed to a decrease in respiratory infections caused by thorough social distancing and mask wearing masks, which strongly suggests that the etiology of KD is related to unidentified respiratory pathogens. This not only reminds us of the importance of ethnic background differences or strengthening respiratory infection control, it suggests that KD can be triggered by more diverse pathogens rather than a single causative pathogen. Table 7 summarizes case reports of SARS-CoV-2 infection as a KD-related pathogen and studies of its association.
Conclusion
If the etiology of a disease is unclear, it obscures its prevention, diagnosis, and management. KD can cause serious sequelae in young children in particular; therefore, continuous efforts to determine its etiology are essential. Thus far, the virus has attracted great attention as one etiology of KD, but it remains difficult to identify a single causative pathogen. The role of the virus in KD pathophysiology or identification of the virus as a direct etiology is expected to be clarified through the development of more advanced diagnostic technologies in the future. However, there is no significant disagreement regarding the pathophysiology of KD in that infection with various pathogens, including viruses, triggers a strong inflammatory response in individuals from certain genetic backgrounds. Therefore, in the future, it will be necessary to conduct continuous research on immune responses in patients with KD, including their genetic backgrounds, and identify KD causative factors, including various pathogens.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.