Haploinsufficiency A20 misdiagnosed as PFAPA (periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis) syndrome with Kikuchi disease
Article information
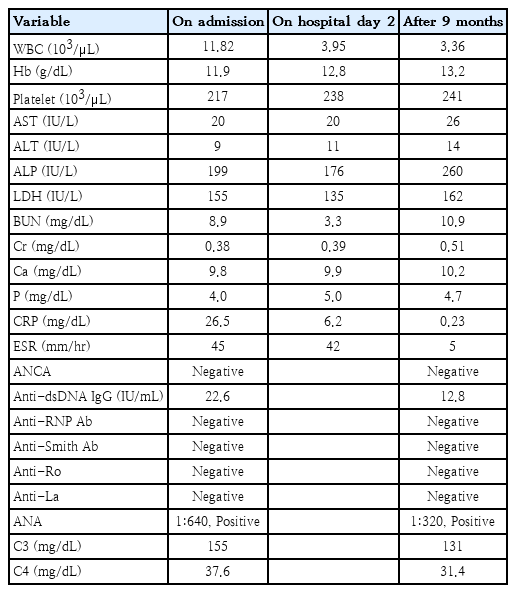
A 9-year-old girl presented with a 10-day history of fever and tender cervical and right axillary lymphadenopathy. The patient had been experiencing periodic fever and oral and genital ulcers since the age of 1 month. Initially, the fever recurred every 1 or 2 months; however, after tonsillectomy at 5 years of age, it recurred every 3 or 4 months. A high fever suddenly occurred at the onset of each episode, and antipyretics decreased body temperature but high fever occurred again during episode. The peak fever temperature was 40°C, and the duration was 4–5 days. During fever, white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels became elevated. Antibiotic treatment was ineffective. She experienced diarrhea during fever and a skin rash appeared after the resolution of fever. Initially, we suspected Kikuchi disease with periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome because, during the current episode, the fever had lasted for 10 days accompanied by tender lymphadenopathy. Initial laboratory test findings showed that the WBC count, ESR, and CRP levels were increased (Table 1).
Q: Which of the following is not a common symptom of PFAPA syndrome?
(1) Periodic fever
(2) Adenitis
(3) Rash
(4) Aphthous stomatitis
On the first day of admission, the fever disappeared without intervention and the WBC count, ESR, and CRP levels decreased. Axillary lymph node biopsy was performed, which demonstrated necrotizing lymphadenitis suggestive of Kikuchi disease. Moreover, an anal ulcer developed. We considered the possibility of other diseases that could cause recurrent fever and oral, genital, and anal ulcers. Anal or genital ulcers are rare in PFAPA syndrome. Detailed family history revealed that her father had recurrent fever and tonsillitis at preschool age. However, he did not have a history of recurrent diarrhea or arthritis.
Next-generation sequencing (NGS) for genes related to periodic fever and autoinflammatory syndromes was conducted to identify a possible genetic disorder. It revealed a nonsense mutation in the tumor necrosis factor (TNF)-α-induced protein 3 gene, leading to the diagnosis of haploinsufficiency A20 (HA20) (Fig. 1). We recommended NGS for her parents and younger sister, but her parents refused. After the diagnosis of HA20, an ophthalmic examination was performed and no uveitis was observed. Colchicine was started, and 9 months after treatment, oral and genital ulcers disappeared. However, the fever continued to recur every 3 or 4 months. Currently, we are considering treatments with immune suppressants such as steroids and biologics.
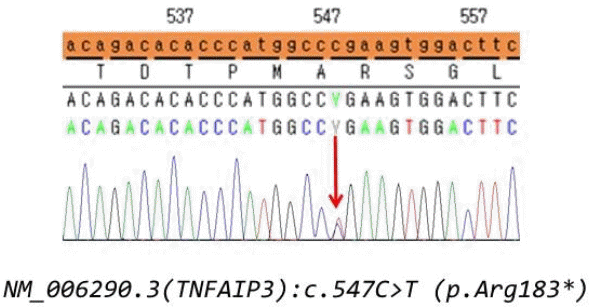
Sequencing analysis of the TNFIP3 gene. The c.547C>T mutation of the proband was detected in the patient. c.547C>T, substitution, position 547, C➞ T; p.Arg183*, nonsense mutation at Arg-183; TNFAIP3 , tumor necrosis factor-alpha-induced protein 3.
What is different with PFAPA syndrome and HA20?
The diagnosis of PFAPA syndrome is established on the basis of clinical criteria [1]. To make a diagnosis of PFAPA syndrome, it is necessary to rule out the presence of another group of diseases that are caused by mutations of genes involved in the regulation of the inflammatory response [2]. Criteria for PFAPA syndrome can distinguish between PFAPA and hereditary recurrent fever syndromes with high sensitivity and specificity [3]. PFAPA is diagnosed when 7 of the following 8 features are satisfied: presence of pharyngotonsillitis, duration of fever between 3–6 days, cervical lymphadenitis, or periodicity; and absence of diarrhea, chest pain, skin rash, or arthritis. Our patient had recurrent fevers and oral, anal, and genital ulcers. In addition, she experienced diarrhea during fever and skin rash after its resolution. These features were atypical for PFAPA syndrome; therefore, we performed NGS to identify inflammatory diseases that cause periodic fever.
According to a review article that included 89 patients with HA20, the most common symptom was oral ulcers (70%), followed by recurrent fever (42%), gastrointestinal ulcers (40%), skin lesions (38%), genital ulcers (36%), musculoskeletal disorders (34%), and autoimmune thyroid disorders (19%) [4]. Abdominal symptoms such as abdominal pain, digestive ulcers, vomiting, diarrhea, and constipation are also common in HA 20 patients [5,6]. The symptoms of our patient were oral, genital, and anal ulcers, recurrent fever, diarrhea, and skin lesions. The c.547C>T variation of the proband was present in our patient, and the TNFAIP3 (c.547C>T, p.Arg183*) gene mutation is already known to cause HA20 [4,6,7].
How to treat HA20?
According to a review article that included 26 studies on HA20, nearly half of the patients responded well to colchicine, especially when used in combination with immunosuppressive or biological agents [5]. In case of worsening of our patient’s symptoms, the next step will be to treat her with biological agents such as TNF-α blockers.
In what cases should genetic testing be considered in patients with periodic fever?
In the present case, Kikuchi disease occurring with PFAPA syndrome was considered initially, but the patient did not have typical symptoms of PFAPA syndrome. Therefore, we conducted NGS for genes related to monogenic autoinflammatory diseases that could cause periodic fever and diagnosed HA20. Until now, only 2 patients, including our case, have been diagnosed with HA20 in Korea [8]. Performing NGS in patients with periodic fever and atypical features of PFAPA syndrome could increase the number of patients diagnosed with HA20. In conclusion, NGS can be used when patients with recurrent fever have atypical symptoms of PFAPA syndrome. In the case of HA20, it can be suspected when there is diarrhea or mucosal ulcerations in patients with recurrent fever, especially when the onset is at a very young age.
Answer: 3
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Ethics approval
This study was approved by the Institutional Review Board (IRB) in Pusan National University Yangsan Hospital (IRB No. 05-2021-274). The authors obtained informed consent from the parents for publish this study.
Funding
This study was supported by a 2022 research grant from Pusan National University Yangsan Hospital.