Graves’ disease: an uncommon cause of late sequelae following DRESS (drug reaction with eosinophilia and systemic symptoms)
Article information
A 14-year-old Thai boy with history of bilateral vesicoureteral reflux diagnosed at the age of 1 year. Then, he progressed to chronic kidney disease and secondary hypertension, respectively at his 6 years of age. Routine medication was enalapril (0.5 mg), (2-tab oral twice a day). He was prescribed allopurinol (100 mg), (1-tab oral once daily) after the diagnosis and persistent of hyperuricemia 13.2 (3.5–7.2 mg/dL) at almost 14-year-old. Forty-five days later, he developed fever and erythematous rash which started on both legs and progressed to entire body within 6 days. There was no other over the counter and routine medication. Allopurinol was discontinued after the rash appeared.
Physical examination showed centrofacial edema, small vesiculopustular rash on patient’s face and neck and erythematous edematous confluent maculopapular rash on trunk and extremities. There was no conjunctival injection or mucosal involvement. Left cervical lymph nodes were palpable, size 1.5 cm×2 nodes. Laboratory revealed hemoglobin 13.5 g/dL, WBC 11.5 ×103/μL, eosinophil 5% (absolute eosinophil count 576), and elevated aspartate aminotransferase, 136 U/L, alanine transaminase, 239 U/L. Drug reaction with eosinophilia and systemic symptoms (DRESS) was diagnosed from allopurinol as the suspected culprit drug by the Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) criteria, including fever, lymph node enlargement, transaminitis, and eosinophilia. He received pulsed intravenous methylprednisolone (1 g/day: 30 mg/kg/day, equal to prednisolone 800 mg) for 6 days, then oral prednisolone 60 mg initially with tapering until 20 mg daily in 4 weeks. HLA B*58:01 was positive. The liver enzymes gradually returned to normal range within 6 weeks and the rash became less edematous, yet more hyperpigmentation.
Two months later, he developed erythroderma with progressive confluent erythematous maculopapular eruption. Relapsed DRESS was suspected. So, he received an increased oral prednisolone to 60 mg daily with gradual tapering by 5 mg/wk until 40 mg daily. He then had a 2-week history of dyspnea and tremor on his hands, with a sudden onset of fever for 1 day prior to the second admission. Physical examination revealed body temperature 37ºC, heart rate 160/min, SpO2 94%, subcostal retraction with fine crepitation on both lower lung zones. Chest x-ray revealed bilateral ground glass opacity.
During PICU stay, he had persistent tachycardia (135–155/min) with fine tremor. Thyroid function test yielded TSH (<0.0083 uIU/mL), free thyroxine (FT4) (2.14 ng/dL; 0.8–2.0), and free triiodothyronine (FT3) (3.05 pg/mL; 2.9–5.6). Thyroid storm was diagnosed with total Burch-Wartofsky Point score of 45. He was prescribed hydrocortisone (100 mg IV q 8 hours: 300 mg/day), methimazole (5 mg), (5-tab oral OD, 0.3 mg/kg/day), and propranolol (40 mg), (1-tab oral q 6 hours, 0.8 mg/kg/dose).
The evaluation of hyperthyroidism revealed positive antithyroid stimulating hormone receptor (anti-TSHR) of 17 IU/L (0–1.75), antithyroglobulin antibody (anti-T) of 371 IU/mL (<115), and antithyroxine peroxidase antibody (anti-TPO) of 36.6 IU/mL (<34), which suspected of having Graves’ disease. The summation for the test result of this patient was shown in Table 1.

Test results of this patient at baseline, upon the DRESS diagnosis, and upon the Graves’ disease diagnosis
After a 5-day course of hydrocortisone (300 mg/day) for thyroid storm treatment, prednisolone was restarted at 60 mg daily with tapering by 5 mg every 2 weeks. Subsequent thyroid function test 3 weeks later showed FT4 0.58 (0.7–1.48 ng/dL), FT3<1.50 (1.6–4.0 pg/mL) and TSH<0.0083 (0.35–7.94 uIU/mL). Methimazole was discontinued due to the possibility of over suppression.
Incidence and clinical manifestation of DRESS
The estimated risk of DRESS syndrome is about 1:1,000 and 1:10,000 [1,2]. The causative agents include anticonvulsants, antimicrobial, antiviral, allopurinol, antihypertensive, antidepressant, and nonsteroidal anti-inflammatory drugs (NSAIDs) [1].
The presumptive pathophysiologic mechanisms of DRESS are complexity. CD4+, CD8+, Tregs, and monomyeloid cells are involved in DRESS drug-specific T cells, plasmacytoid dendritic cells, and monocytes. Dermal dendritic cells could produce chemokine, such as CCL17/TARC, which could potentially attract Th2 lymphocyte to the skin [2,3].
IL-5, IL-4, and IL-13 are produced by Th2 T cells, innate type 2 lymphocytes, and CD4+ T cells, respectively. The function of IL-5 is eosinophil-specific differentiation to develop eosinophilia [3].
Our patient had multiple risk factors, such as time-related factors about 6 weeks after commencing, genetic factors of HLA B*58:01, and drug-concentration factors of initial dose 100 mg, and chronic kidney disease [2,4].
Dermatologic manifestations are variable, including diffuse pruritic macular, erythroderma, polymorphous eruption, and exfoliative dermatitis. Morbilliform rash is the most common presentation [1]. Edema of the face and periorbital area is also reported in approximately 25% of the cases [5].
Liver is the most frequently involved internal organ, followed by hematology, lymphatic system, renal, pulmonary, and cardiac involvements [1].
Endocrinology sequelae of DRESS
There are a few reports of concurrence between diabetes mellitus and thyroid dysfunction with or without viral reactivation in the same patient with DRESS. The 2 most common thyroid dysfunctions are Hashimoto’s thyroiditis and Graves’ disease [6].
The pathophysiologic mechanisms of autoimmune thyroid disease in DRESS are unclear. A combination of genetic susceptibility and environmental factors, such as viral infection, potentially is likely to cause immune dysregulation [7]. Multiple and complex mechanisms are proposed for pleiotropic cytokines releasing, while alteration of cytokines after DRESS could affect the absence or development of autoimmune thyroid disease [7].
There are diversities of thyroid dysfunction onset after DRESS syndrome diagnosis, ranging from 0–36 months in the adult patients [6]. Drug-induced thyroid dysfunction possibly develops up to 53 months after the diagnostic onset [8].
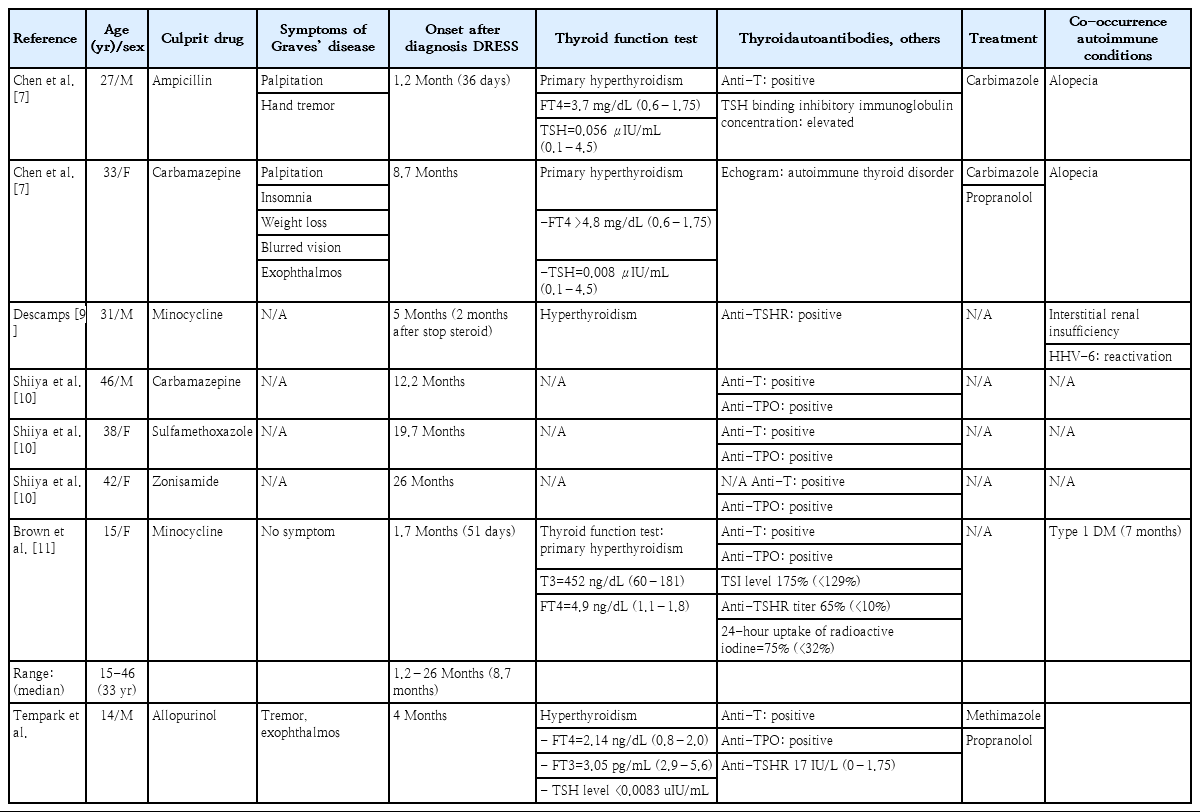
The patients who develop Graves’ disease similar to our patient after DRESS diagnosis in the English literature were summarized in Table 2 [7,9-11]. The majority of culprit drugs were antibiotics. The onset of Graves’ disease was 1.2–26 months (median, 8.7 months). The minority of these patients had clinical symptoms of hyperthyroidism and co-occurrence autoimmune conditions.
Patient outcome
In our case, the patient clinically had hyperthyroidism combined with slightly high FT4 and low TSH at 4 months after DRESS diagnosis with positive antithyroid antibodies. Methimazole and propranolol was prescribed, and he was rapidly recovered within 3 weeks. However, a thyroid function test should be followed and closely monitored for several years.
In conclusion, the screening and monitoring of thyroid dysunction and autoimmune conditions should be strongly recommended for several months after the onset of DRESS symptom.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: TT, AT; Data curation: TT, AT; Formal analysis: TT; Funding acquisition: TT; Methodology: TT, AT; Project administration: TT; Visualization: TT, AT, TD, SC, VS, SW; Writing - original draft: TT, AT; Writing - review & editing: TT, AT, TD, SC, VS, SW
Acknowledgements
The authors would like to thank Ms. Sunattee Kessung for her assistance in editing and revising this manuscript.