Mediation effect of cord blood cortisol levels between maternal prepregnancy body mass index and birth weight: a hospital-based cross-sectional study
Article information
Abstract
Background
Changes in maternal weight affect the maternal and fetal hypothalamic-pituitary-adrenal axis, influencing birth weight and contributing to the fetal origin of adult diseases (Barker’s hypothesis). This study primarily focused on cord blood cortisol levels and identified the association between maternal prepregnancy body mass index (pre-BMI) and birth weight. It also assessed cord blood lipid profile changes related to maternal pre-BMI, birth weight, and cord blood cortisol levels.
Purpose
To study the mediation effect of cord blood cortisol level between maternal pre-BMI and birth weight and its correlation with cord blood lipid profile.
Methods
A total of 169 maternal-neonatal pairs were included at 2 tertiary care centers. Mediation analysis was used to estimate the extent of the association between maternal weight changes and birth weight.
Results
For each unit increase in maternal pre-BMI, birth weight increased by 90.5 g; for every kilogram increase in gestational weight, birth weight increased by 128.44 g. No considerable mediation effect of cortisol was found between pre-BMI and gestational weight gain or between rate of weight gain and birth weight. Pre-BMI and birth weight had a significant negative correlation with high-density lipoprotein cholesterol (HDL-C) levels, i.e., HDL-C was decreased by 1.1 mg/dL for every unit increase in BMI (P=0.017) and for every 100-g increase in birth weight, HDL-C decreased by 0.6 mg/dL (P=0.046). A significant positive correlation was found between cord blood lipid profile and cortisol levels, especially HDL-C (P=0.041).
Conclusion
Cord blood cortisol levels did not mediate the association between maternal weight change and birth weight. A positive correlation was noted between cord blood cortisol levels and HDL-C level. Cord blood HDL-C level was negatively correlated with maternal pre-BMI and birth weight.
Key message
Question: What is the association between cord blood cortisol and maternal weight, birth weight, and cord blood lipid profile?
Finding: Cord blood cortisol levels did not influence the relationship between maternal weight changes or birth weight. Maternal weight changes, birth weight, and cortisol levels altered the cord blood lipid profile.
Meaning: Our findings may aid United Nations Sustainable Development Goal 3 (Good Health and Well-Being) achievement by 2030.
Graphical abstract
Introduction
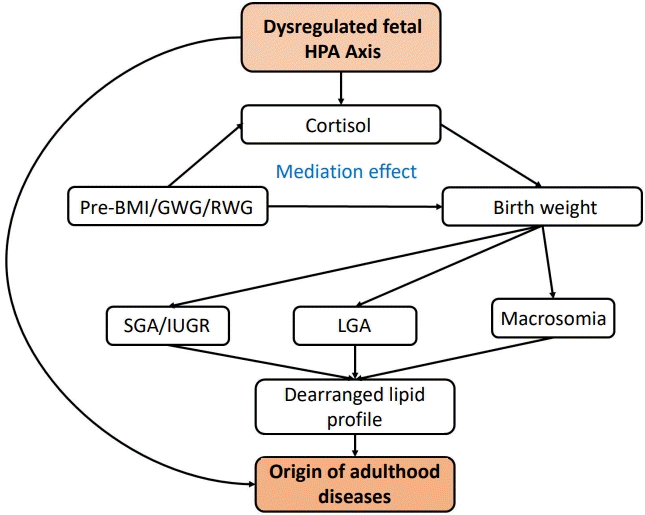
Birth weight is a vital determinant of the health status of the community. Low birth weight (LBW), as well as macrosomia, have implications on the newborn’s survival and health [1]. Macrosomia is associated with obstetric and neonatal complications along with obesity in later life [2]. Extremes of birth weight are associated with chronic diseases which occur later in life, such as the increased risk of type 2 diabetes, hypertension, cardiovascular diseases, and neurocognitive impairment (‘‘Fetal Origin of Adulthood diseases - Barker’s hypothesis”) [3].
Prepregnancy body mass index (pre-BMI) and gestational weight gain (GWG) are the most critical influencers of birth weight [4]. GWG, more or less than recommended by the Institute of Medicine (IOM) guidelines, produces a deleterious effect on the growing fetus [5,6].
Maternal prepregnancy overweight and excessive GWG are associated with macrosomia/large for gestational age (LGA) babies. In contrast, maternal underweight/poor weight gain is related to preterm/LBW/small for gestational age (SGA) babies [7]. However, the exact mechanism behind this is poorly understood.
Recently, studies have shown that changes in maternal weight determine the level of functioning of the maternal hypothalamicpituitary-adrenal (HPA) axis, which is tightly coupled to the fetal HPA axis. Obesity causes hypofunction of the maternal HPA axis which results in hypofunction of the fetal HPA axis leading to a decrease in glucocorticoid levels and undernutrition has the opposite effect. This is depicted by low salivary cortisol levels in overweight pregnant women [8-10].
Cortisol, the most abundant glucocorticoid during pregnancy, accounts for 95% of glucocorticoid activity [11,12]. It is crucial for fetal growth and organ maturation. Cortisol is the result of the HPA axis (corticotropin releasing hormone released from the hypothalamus stimulates the anterior pituitary to secrete adrenocorticotropic hormone which in turn stimulates the adrenal cortex to release cortisol). Hence, it is the primary indicator of the HPA axis function.
The study of lipid profile in LBW/LGA babies at birth is essential because it is linked to metabolic syndrome occurring in later life [13]. The lipid profile in SGA and LGA/macrosomic babies is deranged compared to appropriate for gestational age (AGA) babies. This is related to maternal pre-BMI and GWG [14-16].
There is insufficient evidence to point out the relationship between maternal weight changes, birth weight and steroid hormone metabolism. Furthermore, the reason behind the altered lipid profile is not well understood. Therefore, this study was focused to determine the influence of maternal pre-BMI/GWG/rate of weight gain (RWG) on the birth weight through the measurement of cord blood cortisol levels. Secondly, the changes in cord blood lipid profile concerning the maternal pre-BMI, birth weight, and cord blood cortisol level were investigated.
The study flow (Fig. 1) depicts what we intend to do in this study and what we would like to emphasize.
Methods
1. Ethics
Written informed consent was obtained from all study participants and the research approval was obtained from the Institutional Ethics Committee of Kasturba Medical College, Mangalore (IEC KMC MLR 10-19/492).
2. Study population
This study was conducted at 2 tertiary care centers and teaching hospitals affiliated with Kasturba Medical College, Mangalore, located in the state of Karnataka, the southern part of India. The study included the maternal – neonatal pair admitted during the study period (September 2019–September 2021). Primigravida with single intrauterine gestation and term neonates delivered via normal vaginal delivery were included in the study. Mothers with underlying comorbidities and no documented anthropometric values during pregnancy as well as neonates requiring resuscitation or neonatal intensive care unit stay were excluded from our study.
3. Data collection
Prepregnancy weight, gestational age from first-trimester ultrasonography and underlying illnesses (e.g., gestational, and overt diabetes, hypertension-chronic and pregnancy-induced, thyroid disorders) were obtained from medical records. Maternal height was documented during their first-trimester visit using a stadiometer through a standard measurement technique. Body mass index was calculated and interpreted using the World Health Organization BMI chart for the Asian population [17]. The date and time of birth of the newborn were obtained from neonatal records. Birth weight was measured in grams using an electronic weighing machine after calibrating to zero. Gestational age documented in the maternal records was confirmed using New Modified Ballard’s score to include only term neonates [18].
4. Categorization
With the help of pre-BMI, the study population was grouped into 3 groups-underweight (BMI<18.5 kg/m2), normal (BMI=18.5–24.9 kg/m2) and overweight (BMI≥25 kg/m2) groups. GWG in every trimester was obtained from the antenatal records, and we calculated the total GWG (GWG-T) and RWG during pregnancy, then categorized using American College of Obstetricians and Gynaecologists/IOM guidelines [19] into 3 groups, GWG less than or more than or equal to IOM recommendations (GWG<IOM, GWG>IOM, and GWG= IOM).
According to gestational age and birth weight, the babies were classified as small/appropriate/large for gestational age (SGA/AGA/LGA) using Lubchenco's charts [20]. Birth weight below 2,500 g was considered LBW, and above 4,000 g was considered macrosomia.
5. Cord blood cortisol and lipid profile assay
Cord blood was obtained from newborns in the delivery room after enrolling the mothers in the study with written informed consent. Blood was collected from the umbilical vein using a 22G needle and syringe under sterile aseptic precautions. Serum was obtained after centrifugation at 4.000 rpm and was stored frozen in micro vials at -60 degrees centigrade for analysis. Serum cortisol was measured using the DRG Cortisol ELISA kit. Lipid levels of total cholesterol (TC), HDL-C, triglycerides (TG) were analyzed using an auto-analyzer, and low-density lipoprotein cholesterol (LDL-C) and very-low-density lipoprotein cholesterol (VLDL-C) were mathematically calculated using the Friedwald formula. A total of 169 samples were collected and among the 169 lipid samples processed, 51 (30%) values had to be discarded from the study. Because the Friedwald formula has its limitations at very low LDL-C or very high TG concentrations, resulting in negative LDL-C values and as there are no reference levels for cord blood lipid concentration have been established in the literature, those values had to be discarded for the sake of reliability, comparability, and consistency of our results [21]. One hundred sixty-nine cord blood cortisol and 118 cord blood lipid levels were analyzed.
6. Statistical analysis
We represented the continuous variables, i.e., cortisol and lipid levels, by median and interquartile range, and categorical variables using frequency and percentage. After stratifying the variables into 3 groups based on pre-BMI and GWG/IOM, their differences were tested using 1-way analysis of variance with Tukey B. Karl Pearson correlation coefficient was used to find the correlation between pre-BMI, RWG, GWG, cortisol, and lipid levels, and their relationship was tested using multiple variable linear regression analysis.
Mediation analysis was conducted to investigate the extent of association between maternal pre-BMI and GWG with birth weight using cord blood cortisol. It was performed with SPSS PROCESS MACRO with cortisol (M variable), pre-BMI/GWG/RWG (X variable), and birth weight (Y variable). Total effect (TE), direct effect (DE) and indirect effects (IE) were estimated. The proportion of mediation is IE divided by TE.
All statistical analyses were done using IBM SPSS Statistics ver. 25.0 (IBM Co., Armonk, NY, USA). A P value of <0.05 was considered statistically significant for a 2-tailed test.
Results
1. Study population
The baseline characteristics of the study population stratified based on pre-BMI are represented in Table 1. We found that the median GWG of the normal BMI group was 12.0 kg (10.0–13.5 kg) with a median age of 26 years (23–29 years). Among the 3 groups, there was a statistically significant difference in total GWG and the RWG. There was a rising trend in GWG and RWG from the underweight group up to the overweight group (P<0.001).
Most of the newborns had a birth weight appropriate for their gestational age. Overall, there was a significant difference in birth weight among the 3 BMI groups, however, there was no statistical significance in birth weight between the normal and overweight groups when looked at it separately (P=0.44). In the overweight group, there was a predominance of LGA babies with a specific predilection for the female sex (0% vs. 13% vs. 26%, P<0.001).
2. Maternal weight changes and birth weight
We observed that for every unit increase in maternal pre-BMI, birth weight increased by 90.50 g (95% confidence interval [CI], 71.019–110.00) (P<0.001), and for each kilogram rise in gestational weight, birth weight increased by 128.44 g (95% CI, 109.761–147.119) (P<0.001) as depicted in Table 2.
3. Maternal weight changes, birth weight, and cord blood cortisol
Cord blood cortisol concentration among the 3 groups was stratified based on pre-BMI and GWG/IOM guidelines. A median value of 381.0 ng/dL (196.0–617.0 ng/dL) in the overweight group and 332.0 ng/dL (192.0–598.25 ng/dL) in GWG> IOM was noted. We observed no significant difference in cortisol levels among the 3 BMI groups (P=0.56) and the GWG/IOM groups (P=0.63). From our study, no significant correlation was found between the maternal pre-BMI, GWG, and the birth weight of the newborn with cord blood cortisol levels.
4. Mediation analysis
Mediation analysis was carried out to determine the proportion of association between maternal pre-BMI, GWG-T, RWG and birth weight by cord blood cortisol concentration. As revealed in Fig. 2, cord blood cortisol had no significant effect on mediation between maternal pre-BMI/GWG/RWG and birth weight. Hence, the proportion of mediation cannot be established. This indicates that cortisol does not mediate the association between maternal pre-BMI/GWG/RWG and birth weight.
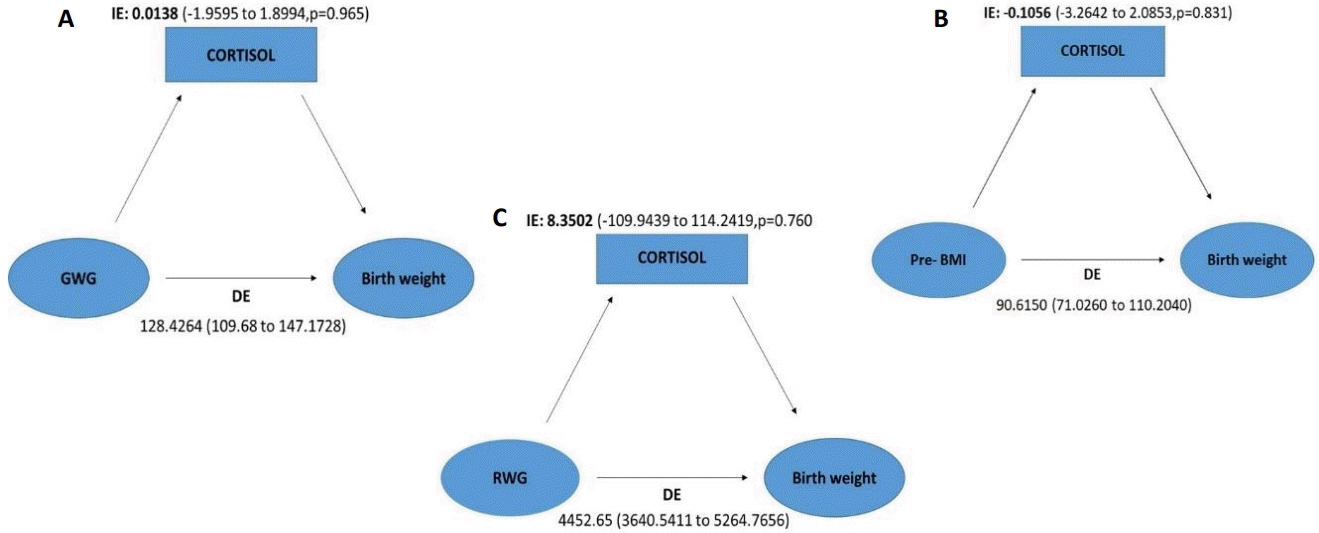
Pictorial representation of mediation analysis of cord blood cortisol effect between maternal weight changes and birth weight. Effect of GWG (A), maternal pre-BMI (B), and RWG (C) on birth weight mediated by cortisol levels in cord blood estimated by direct effect (DE) and indirect effect (IE). GWG, gestational weight gain; pre-BMI, prepregnancy body mass index; RWG, rate of weight gain.
5. Maternal weight changes, birth weight, and cord blood lipid profile
The cord blood lipid levels stratified by pre-BMI and GWG/IOM showed no significant difference among the 3 groups. Maternal pre-BMI (20 kg/m2 [18–23 kg/m2]) and birth weight had a significant negative correlation with HDL-C levels (53 mg/dL [44–63 mg/dL]) as shown in Table 3. That is for every unit increase in maternal pre-BMI, HDL-C is reduced by 1.1 mg/ dL (95% CI, 1.99 to -0.201) (P=0.017), and for every 100-g increase in birth weight, HDL-C decreased by 0.6 mg/dL (95% CI, 0.011–0.0001) (P=0.046)
6. Cord blood cortisol and lipid profile
In our study, a significant positive correlation was found between cord blood lipid levels and cord blood cortisol levels except for LDL-C. For every unit increase in cortisol (340 ng/dL [249–463 ng/dL]), there was an increase in HDL-C, TG, TC, and VLDL-C levels (P<0.05) as depicted in Table 4.
Although changes in lipid parameters with an increase in cortisol were not in the clinically significant range, a positive correlation has been identified signifying cortisol levels play a pivotal role in controlling fetal lipid homeostasis.
Discussion
Our research focused on determining the influence of maternal pre-BMI on the birth weight and lipid profile through the measurement of cord blood cortisol levels. Our study showed that there was no mediation effect of cord blood cortisol levels between maternal pre-BMI/GWG/RWG and birth weight. A significant positive correlation was observed between cord blood cortisol level and cord blood HDL-C. A negative correlation was found between HDL-C levels and both maternal pre-BMI and birth weight.
The literature has well described the association between maternal weight changes and birth weight [22,23]. But the role of steroid hormones in controlling this relationship has not been thoroughly investigated. To the best of our knowledge, this is the first study to determine the effect of cord blood cortisol on the fetal lipid profile.
In our study, we noted that every unit increase in pre-BMI and GWG was associated with an increase in birth weight of 90.50 g and 128.44 g, respectively (P<0.001). Therefore, this emphasizes the importance of maintaining optimum maternal weight gain before pregnancy and during pregnancy [24,25].
Also, there was no significant mediation effect of cord blood cortisol on maternal pre-BMI, GWG, RWG and birth weight from our study. However, we cannot rule out the influence of other steroid hormones in the maternal-placental-fetal unit on the birth weight of the newborn. A study by Jin et al. [12] analyzed all the steroid hormones in the maternal-placental-fetal unit in their large birth cohort, which showed a partial mediation effect of 3.48% with corticosterone and in mothers with GWG>IOM, 4.33% and 5.38% mediation with cortisol and corticosterone respectively.
In our study, cord blood HDL-C levels had a negative correlation with both maternal pre-BMI and with birth weight. Studies by Mandraha et al. [14] and Pac-Kozuchowska et al. [26] have shown similar findings of decreased HDL-C levels in neonates with maternal BMI ≥25 kg/m2. Increased LDL-C, VLDL-C, TG, and cholesterol levels were seen in LBW/SGA and LGA babies but there was no significant correlation with HDL-C levels [16,27]. Furthermore, in this study, the cord blood cortisol had a positive correlation with HDL-C, TC, TG, and VLDL-C levels (P<0.05).
Our findings of decreased HDL-C with increasing maternal weight, and birth weight were consistent with other studies described in the literature [28]. This influence of cord blood cortisol levels, maternal pre-BMI, and birth weight on HDL-C can explain the possibility of a dysregulated maternal-fetal HPA axis. As per our study results, maternal obesity causes hypofunction of the maternal-fetal HPA axis which results in low cortisol levels leading to decreased HDL levels. We can postulate that changes in maternal weight, birth weight, and lipid profile may be due to dysregulated maternal-fetal HPA axis based on our research.
The impact of birth weight on the lipid profile later in life has been noted in a 53-year-old birth cohort from England. This emphasizes the need for an optimal birth weight, which in turn depends on the maternal weight [29]. Our study demonstrated the effect of changes in maternal weight on the birth weight and their effect on HDL-C levels in the fetus. These suggest the need for strengthening interventions to manage maternal weight before as well as throughout pregnancy.
Our study has shown that cord blood cortisol levels did not influence the relationship between maternal pre-BMI, GWG, RWG, and birth weight. However, maternal pre-BMI and birth weight as well as cord blood cortisol levels had a detrimental impact on cord blood HDL-C levels. Also, changes in cortisol levels caused alteration in other cord blood lipid parameters. Therefore, our data imply that early-life exposure to extremes of maternal weight changes may have an impact on birth weight and fetal lipid homeostasis.
We tested cortisol levels in cord blood, but other steroid hormones (such as mineralocorticoids, sex steroids, and other glucocorticoids) were not measured. Serial measurements of cortisol levels after birth were not done. Maternal cortisol and lipid levels were not correlated simultaneously.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
Funded by ICMR MD/MS Thesis Research Grant -MD20JUN-0083 and Manipal Research grant.
Author contribution
Conceptualisation: NS, JK; Data Curation: NS, JK, PM; Formal Analysis: NS, JK, PM; Funding acquisition: NS, JK, From ICMR and Manipal University; Methodology: NS, JK; Project administration: NS, JK, PM; Visualisation: NS, JK; Writing-Original Draft: NS, JK; Writing-Reviewing and editing: NS, JK, PM
Acknowledgements
We would like to thank all the parents for their co-operation and laboratory personnel for their assistance. Special appreciation to Dr Rukmini, Associate Professor, Department of Biochemistry, Kasturba Medical College, Mangalore, for her guidance in sample storage and processing.