Ferritin in pediatric critical illness: a scoping review
Article information
Abstract
This scoping review aimed to elucidate and summarize the predictive role of serum ferritin in critical pediatric illness. The Preferred Reporting Items for Systematic reviews and Meta-Analyses methodology was employed to conduct a scoping review of 5 databases (MEDLINE, CENTRAL, ProQuest, ScienceDirect, and Epistemonikos) from the date of inception through January 24, 2022. Primary research studies involving subjects aged <18 years and serum ferritin levels were screened and reviewed independently following an a priori defined protocol. Of the 1,580 retrieved studies, 66 were analyzed. Summary statistics of serum ferritin levels for overall and condition-specific studies were reported in 30 (45.4%) and 47 studies (71.2%), respectively. The normal range was defined in 16 studies (24.2%), whereas the threshold was determined in 43 studies (65.1%). A value of <500 ng/mL was most often the upper limit of the normal range. Serum ferritin as a numerical variable (78.9%) was usually significantly higher (80.8%) in the predicted condition than in controls, while as a categorical variable with preset thresholds, ferritin was a significant predictor in 84.6% of studies. A total of 22 predictive thresholds predicted mortality (12 of 46 [26.1%]), morbidity (18 of 46 [39.1%]), and specific (16 of 46 [34.8%]) outcomes in 15 unique conditions. Increased precision in serum ferritin measures followed by close attention to the threshold modeling strategy and reporting can accelerate the translation from evidence to clinical practice.
Key message
The number of studies on ferritin predictive ability in pediatric critical illness has grown exponentially over the past 2 decades. However, among the 66 of 1,580 studies analyzed here, summary statistics for overall and condition-specific studies were only reported in 45.4% and 71.2%, respectively. In contrast, ferritin as a categorical variable with a preset threshold was a significant predictor in 84.6% of studies.
Graphical abstract
Introduction
Ferritin is a spherical protein composed of a protein shell that encapsulates an iron core. The shell consists of 24 protein subunits called apoferritin, with a certain ratio of H and L chains according to tissue type. The Fe3+ state of iron is stored in the iron core [1]. This iron storage protein can be found in virtually all cells with cytosolic, mitochondrial, and nuclear localization. Since its discovery in 1937 and quantification in human serum in 1972, the role of ferritin in clinical practice has expanded beyond its well-established function in iron homeostasis [2]. Areas of ferritin utility in diagnostic and prognostic studies have evolved considerably from indicators of iron storage, acutephase reactants, disease biomarkers, and recently discovered hyperferritinemic syndrome [3].
Despite the decreasing trend in the global pediatric mortality rate in the pre-coronavirus disease 2019 (COVID-19) pandemic period, the morbidity rate continued to increase owing to unresolved issues caused by acute illnesses [4]. Pre- and pandemic comparisons of pediatric critical illness trends revealed increasing mortality rates despite concurrent lower critical care admission rates [5,6]. Considering that both acute potentially fatal illness and medical complexity resulting from morbidity are subject to critical care management [7], this evidence suggests the need for evidence-based critical care improvements, especially those that enable the early prediction of critical illness outcomes to prevent mortality and morbidity [8,9].
Ferritin is among the most thoroughly investigated biomarkers for predicting outcomes under various conditions [10-13]. It was also included as a defining criterion for the critical COVID-19 spectrum, specifically in the pediatric population, namely multisystem inflammatory syndrome in children (MIS-C) and pediatric multisystem inflammatory syndrome temporally associated with COVID-19 [14,15]. However, a systematic review of their multifaceted predictive ability is lacking. This systematic scoping review aimed to: (1) identify predictive serum ferritin threshold(s); (2) investigate targeted clinical conditions; and (3) map available outcomes.
Methods
This scoping review followed a previously established methodological framework [16,17] according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews checklist [18]. The a priori study protocol was followed closely to ensure a transparent and systematic approach [19].
1. Sources and search strategy
Searches were conducted in MEDLINE (PubMed), CENTRAL (Cochrane Library), ProQuest, ScienceDirect, and Epistemonikos using combined keywords and controlled vocabulary (pediatric, critical illness, and ferritin) and their synonyms connected with appropriate Boolean operators (AND and OR). The search strategy was initially constructed for PubMed before it was adapted to the other databases (Supplementary Material 1). An additional search for gray literature was performed to locate unpublished studies. Filters for articles written in English and full-text availability were applied where possible, and all search results from the date of inception to January 24, 2022, were included.
2. Study selection
The inclusion criteria were as follows: (1) primary research study; (2) subjects aged <18 years; and (3) serum ferritin measurement results. The exclusion criteria were as follows: (1) secondary research study; (2) nonresearch publication type; (3) noncritical illness or condition; (4) pediatric outcomes not separated from adult outcomes; and (5) ferritin measurement not presented.
The search results from all databases were downloaded as reference lists and combined to check for duplicates using reference management software. After duplicate removal, the combined references were managed using the Rayyan systematic review application. Title and abstract screenings were performed independently by 2 reviewers (ICV and MP) to verify study eligibility and remove the remaining duplicates. Conflicting decision(s) regarding study eligibility were resolved by discussion with a content expert (DKW). A full-text review was performed prior to the data extraction whenever necessary.
3. Data extraction and analysis
Study characteristics (year of publication, first author, country of origin, country income group, study design, setting, objective (s), inclusion and exclusion criteria, study period, number of subjects, age, and sex ratio) were extracted and tabulated. Continent and country were collated according to the study subjects’ country of origin. The country income group was defined according to World Bank classification [20]. Age categories (upper age limit for neonates, 1 month; infants, 2 years; children, 12 years; adolescents, 18 years) were defined based on the baseline characteristics and/or study eligibility criteria [21]. Data related to ferritin measurement (definition, threshold, diagnostic test indices, normal range, proportion, summary statistics) and predicted outcomes (diagnosis or condition, outcome, significance, statistical analysis) were iteratively tabulated to obtain comprehensive data.
A symbol map was created using Tableau Public that can be accessed at https://public.tableau.com/views/FerritinMap/Dashboard1?:language=en-US&publish=yes&:display_count=n&:origin=viz_share_link . Data visualization using horizontal boxplots was performed in Python 3.9.7 using the matplotlib plotting library. The central tendencies were plotted as a vertical line between whiskers representing the upper and lower values of dispersion. Logarithmic transformation with a base of 10 was performed for scaling purposes while retaining the original absolute serum ferritin levels. Hence, negative values of the lower bound of the lower whisker in instances in which the standard deviation was greater than the mean were omitted considering that the actual value should always be positive.
Results
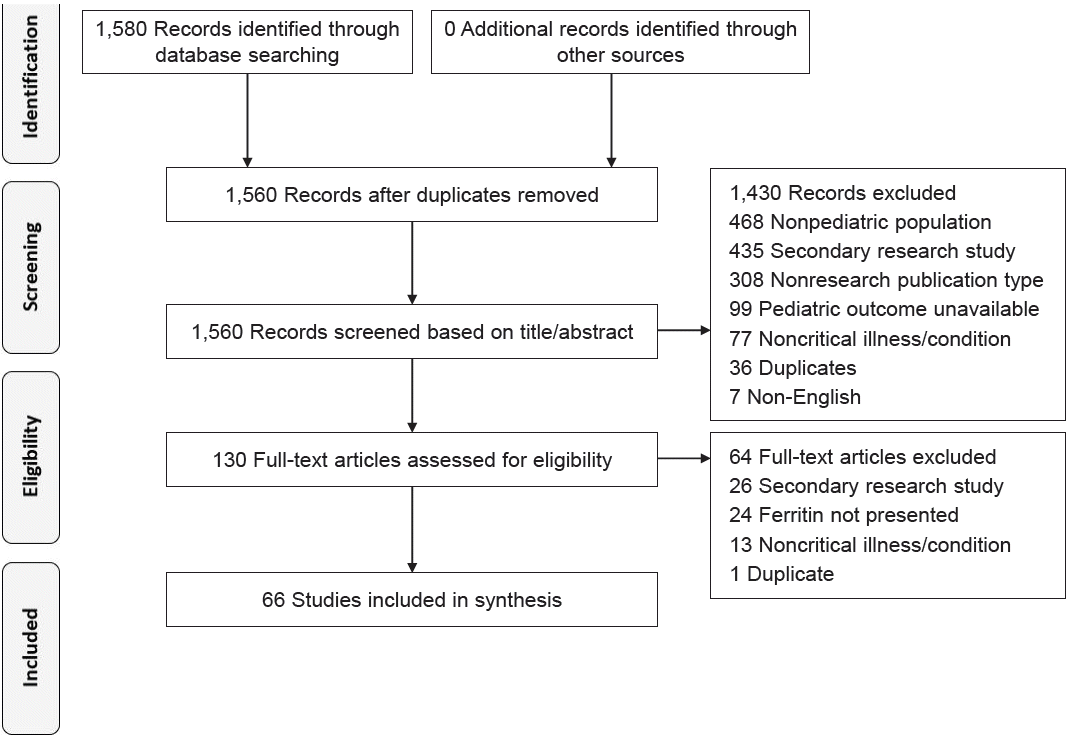
A total of 66 of the 1,580 identified citations were included in the current scoping review (Fig. 1). Complete information about the included studies can be found in Supplementary Material 2. The exponential growth in the number of studies on ferritin has been evident in the last 2 decades. Up to 36.4% of the included studies were published in 2021. Observational (56.1%) and cohort (22.7%) study designs were predominant, while the minority of others (3.0%) consisted of survey and surveillance studies. The number of retrospective studies (50.0%) slightly exceeded that of their prospective counterparts (37.9%). The study settings were as follows: hospital (36.3%), intensive care unit (ICU) (27.3%), multicenter (24.2%) to university facilities (6.1%), and clinics (3.0%). Of the 34 representative countries, most studies were performed in Asia (45.6%), and underrepresentation of Africa (1.5%) was noteworthy. Lower- to middleincome countries were represented by only 14 studies (21.2%) (Table 1).
Authors from the United States (16 [24.2%]), India (9 [13.6 %]), and Turkey (7 [10.6%]) generated nearly half of the studies, whereas most subjects originated from the United States (2,837 [32.4%]), Pakistan (971 [11.1%]), and India (925 [10.6%]) (Supplementary Material 3). More than half (63.6%) of the studies involved fewer than 100 subjects per study. Nonetheless, one multicenter surveillance study involved 1,080 subjects across the United States. Children constituted the largest proportion (77.2%) of the study subjects, even more than the mix of more than 2 pediatric age categories (43.9%). A male preponderance was observed in 48 of 63 studies (76.2%) (Table 1).
1. Summary statistics
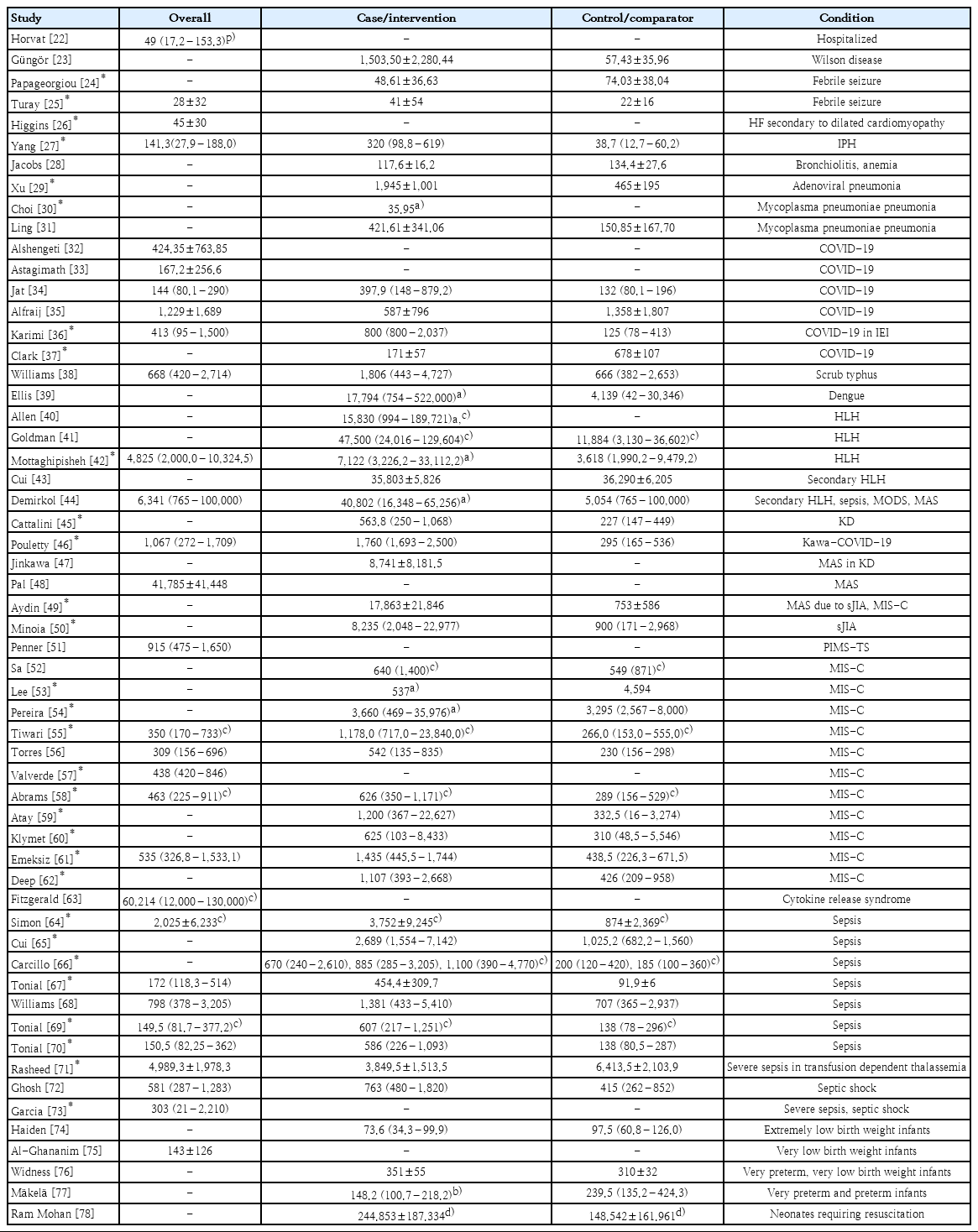
Statistics for overall and condition-specific serum ferritin levels were reported by 30 (45.4%) and 47 (71.2%) of the studies, respectively (Table 2) [22-78]. Although most summary statistics were presented as median (interquartile range) (43.9%) or mean±standard deviation (43.9%), the remaining 12.1% were presented as median with or without range and mean with 95% confidence interval (CI). Baseline values (43.6%) were obtained more frequently than peak values (15.1%) and postintervention values (1.5%).
The overall ferritin summary statistics are shown in Fig. 2. The ranges of overall serum ferritin levels in hospitalized patients, febrile seizures, heart failure (HF), idiopathic pulmonary hemosiderosis (IPH), and very low birth weight (VLBW) infants largely spanned tens and hundreds of nanograms per milliliter. While the ranges of overall serum ferritin in COVID-19, sepsis and septic shock vary widely from hundreds to thousands, their upper extremes coincide with those of MIS-C, scrub typhus, and pediatric multisystem inflammatory syndrome temporally associated with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) mimicking Kawasaki disease (KD; KawaCOVID-19). The overall range for hemophagocytic lymphohistiocytosis (HLH) was gauged at thousands in contrast with the broadly extending ranges of macrophage activation syndrome (MAS) and overlap of secondary HLH/MAS, which extends from thousands to tens of thousands. Cytokine release syndrome had the highest range, encompassing tens of thousands to hundreds of thousands of serum ferritin (in ng/mL).
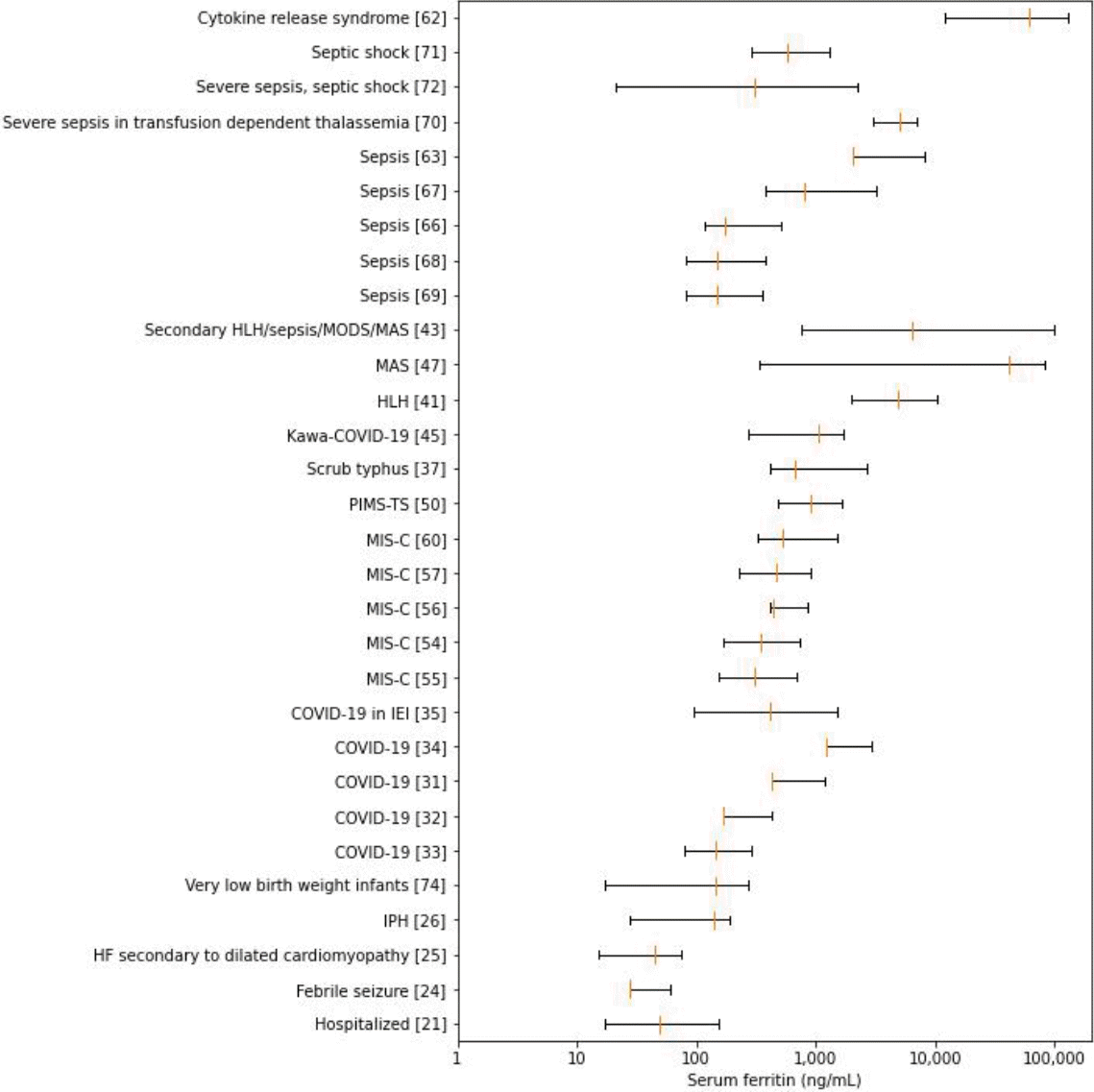
Boxplots of overall serum ferritin summary statistics. HLH, hemophagocytic lymphohistiocytosis; MODS, multiple organ dysfunction syndrome; MAS, macrophage activation syndrome; COVID-19, coronavirus disease 2019; PIMS-TS, pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2; MIS-C, multisystem inflammatory syndrome in children; IEI, inborn errors of immunity; IPH, idiopathic pulmonary hemosiderosis.
Serum ferritin levels in the case and intervention groups are presented in Fig. 3. The ranges for complex febrile seizure and extremely low birth weight infants were within tens of nanograms per milliliters. The ranges gradually shifted to the right within hundreds in very preterm, very preterm, and VLBW infants, overarched by neonates requiring resuscitation. Respiratory conditions such as bronchiolitis, IPH, and Mycoplasma pneumoniae pneumonia (MPP) with hypoxia and sepsis spectrum were within hundreds of nanograms per milliliter, while adenoviral pneumonia along with fulminant Wilson disease and scrub typhus showed greater values at thousands of nanograms per milliliter. Data on dispersion were not available for MPP, and the central tendencies of refractory MPP and MPP with hypoxia were far apart. The figure for COVID-19 spread across hundreds to thousands, which overlapped MIS-C, with the exception of further extension to tens of thousands in the latter. Systemic juvenile idiopathic arthritis (sJIA) before and after MAS, MAS in KD, and Kawa-COVID-19, although inconsistently, spanned thousands of serum ferritin levels. Notably, primary and secondary HLH and dengue-associated HLH extended from thousands to hundreds of thousands.
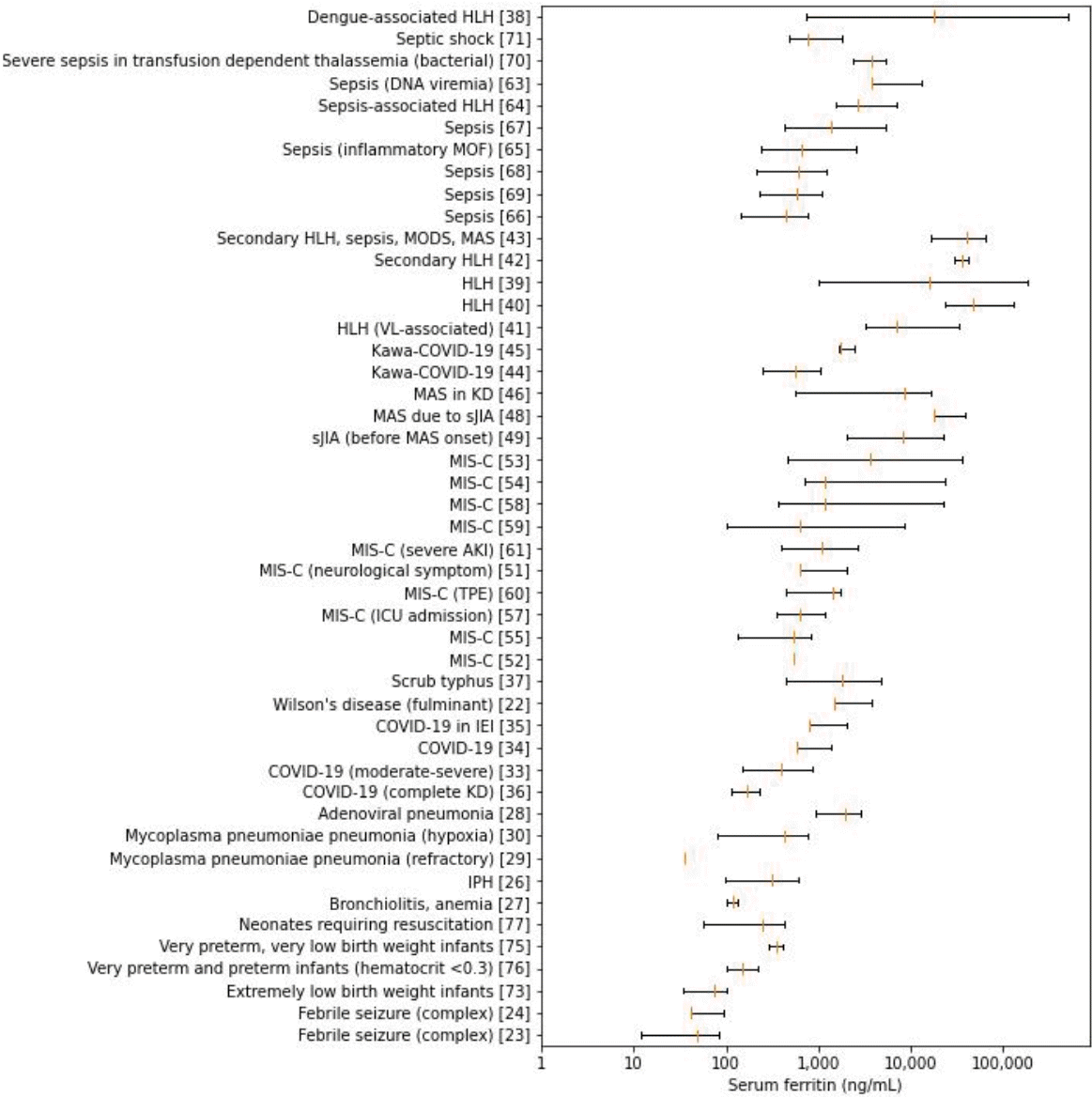
Boxplots of serum ferritin summary statistics in the case or intervention group. HLH, hemophagocytic lymphohistiocytosis; VL, visceral leishmaniasis; MOF, multiorgan failure; MODS, multiple organ dysfunction syndrome; MAS, macrophage activation syndrome; COVID-19, coronavirus disease 2019; KD, Kawasaki disease; sJIA, systemic juvenile idiopathic arthritis; MIS-C, multisystem inflammatory syndrome in children; AKI, acute kidney injury; TPE, therapeutic plasma exchange; ICU, intensive care unit; IEI, inborn errors of immunity; IPH, idiopathic pulmonary hemosiderosis.
2. Normal ranges and thresholds
The normal range of serum ferritin level was defined in 16 studies (24.2%), while its threshold was predetermined in 32 studies (48.5%) and determined by analysis in 11 studies (16.7%). A value of less than 500 ng/mL was most often considered the upper limit of normal (5 of 16 [31.2%]) and threshold (11 of 43 [25.6%]). Only 2 studies specified demographic-specific normal range values. A wide variation in serum ferritin threshold was observed within and between different conditions or diseases of interest. The proportions of increased, normal, and decreased serum ferritin levels were reported in 25 (37.9%), 6 (9.1%), and 7 studies (10.6%), respectively. Iron status was more often defined as a decreased serum ferritin level (57.1%) than an increased serum ferritin level (4%). An increased serum ferritin was used to indicate hyperferritinemia (16%), and the diagnosis or disease activity (12%) was almost equal (Table 3) [75-87].
3. Predictive ability
Among the 55 studies (83.3%) analyzing serum ferritin predictive ability against outcomes, 45 (81.8%) yielded significant results. Thirty of 38 analyses (78.9%) treated serum ferritin as a numerical variable, while 11 of 13 analyses (84.6%) treated it as a categorical variable with a preset threshold. Serum ferritin levels in the case or intervention group were generally (80.8%) higher than those in the control or comparator group. A significant relationship was found with mortality (12 of 46 [26.1%]), morbidity (18 of 46 [39.1%]), and specific (16 of 46 [34.8%]) outcomes in ICU patients (3,000 ng/mL), VLBW infants (500 ng/mL), febrile seizure, HF secondary to dilated cardiomyopathy, IPH, adenoviral pneumonia, MPP (171.15 and 174.15 ng/mL), COVID-19 in pediatric oncology (1,000 ng/mL) and inborn errors of immunity, HLH (10,000 ng/mL), KD, KawaCOVID-19, MAS (50,000 ng/mL), sJIA, MIS-C (500 ng/mL and per 100-unit increase), sepsis (300 and 500 ng/mL), severe sepsis, dengue-associated HLH (500 ng/mL), and allogeneic hematopoetic cell transplantation (1,000 ng/mL). Diagnostic test indices reported as area under the curve (AUC; 95% CI), sensitivity, and specificity achieved significance in hospitalized patients (373 ng/mL; AUC, 0.88; 95% CI, 0.79–0.97), Wilson disease (93.5 ng/mL; 95% CI, 0.773–0.989; 92.9%, 66.2%), MPP with hypoxia (171.15 ng/mL; AUC, 0.806; 82.4%, 69.3%), and refractory (230 ng/mL; 67%, 67%), Kawa-COVID-19 (1,400 ng/mL; AUC, 0.957; 80%, 100%), sepsis (135 ng/mL; AUC, 0.785; 95% CI, 0.733–0.830; 96%, 49%; 500 ng/mL; AUC, 0.767; 95% CI, 0.633–0.900); 1,210 ng/mL; AUC, 0.89; 95% CI, 0.74–1.04; 88%, 85%), severe sepsis (500 ng/mL; 64%, 80%; 1,980 ng/mL; AUC, 0.878; 95% CI, 0.751–1.000; 75%, 92%), and HLH (10,000 ng/mL; AUC, 0.92; 95% CI, 0.88–0.95; 90%, 96%) (Supplementary Material 2).
Discussion
The increasing burden of pediatric critical illness necessitates advancements in mortality and morbidity outcome predictions [88-91]. A growing body of evidence supports the potential use of ferritin as a biomarker in multiple clinical contexts. However, without careful interpretation, its equivocal nature can inevitably confound its predictive ability [92]. Therefore, this study aimed to identify the prospects and gaps in interpreting serum ferritin levels in different clinical conditions. A total of 22 predictive thresholds for 15 unique conditions were identified.
1. Outcome predicting thresholds
Considering the statistical significance and baseline serum ferritin measurements, thresholds for predicting hypoxia in MPP (174.15 ng/mL) [31], severe acute kidney injury (AKI) in MIS-C (per 100-unit increase) [62], fewer ventilation-free hours and higher maximum inotropic score (300 ng/mL) [67] and HLH (500 ng/mL) [65] in sepsis, mortality in severe sepsis (1,980 ng/mL) [85], and stage-3 AKI in allogeneic hematopoietic cell transplantation (1,000 ng/mL) [86] are outcome-defining thresholds for predictions upon admission. Peak serum ferritin thresholds for surgical ligation of patent ductus arteriosus, sepsis, and moderate or severe states of bronchopulmonary dysplasia in VLBW infants (500 ng/mL) [80], DNA viremia in sepsis (1,210 ng/mL) [64], and mortality in hospitalized patients (373 ng/mL) [22], ICU admitted patients (3,000 ng/mL) [87], and HLH (10,000 ng/mL) [41] are more suitable for monitoring purposes.
2. Study characteristic representation
Descriptive analysis revealed the underrepresentation of several key demographic features. Age and sex cause significant differences in the serum ferritin reference range, even within an apparently healthy pediatric population [93,94]. Most reviewed studies included mixed age categories and predominantly included men. Appreciation of these features was considered in only 2 studies that provided age- and sex-specific normal ranges. Meanwhile, no such specification has been reported for serum ferritin thresholds. The disparity in studies’ countries of origin has 2 indirect implications, namely, ethnicity and country income. While ethnicity-related genetic variants are reportedly associated with serum ferritin differences [95], the paucity of data from lowerto middle-income countries can lead to disease burden underestimation, thus impeding diagnostic and therapeutic improvement in resource-limited settings where it is needed the most. These issues can be addressed by adjusting the key demographic features according to study design or analysis and specific reporting of serum ferritin levels.
3. Methods and translation into clinical praxis
The first concern was the wide range of serum ferritin levels that were evident between conditions as well as studies on the same condition. Nevertheless, this phenomenon is readily observed in normally distributed data that reported a standard deviation greater than the mean. The skewed normal distribution is a plausible explanation for this finding, which was at least partially due to the small sample size. This was confirmed in the current review, in which 63.6% of studies included fewer than 100 subjects. Skewness rendered the mean and standard deviation unrepresentative of the actual central tendency and dispersion [96]. Hence, improved precision by increasing of the sample size in future studies is imperative to generate the true serum ferritin threshold.
Unjustified thresholds (74.4%) were 3 times more common than justified thresholds (25.6%). Even among those justified by the analysis, methods to determine optimal threshold values were mentioned in only 3 of 11 studies (27.3%). All 3 studies used the Youden index derived from the sum of the sensitivity and specificity subtracted by 100%. The importance of justifying the methods cannot be overstated, especially in critical conditions when decision-making strategies become the cornerstone of the outcome. Various approaches are available to determine the optimal threshold from the receiver operating characteristic analysis [97]. Furthermore, the integration of outcome probability, cost of misprediction, and patients’ values and preferences in advanced modeling can accelerate the substantiation of evidence-based clinical practice.
4. Mechanistic underpinnings
Finally, the vast growth of serum ferritin diagnostic studies requires balancing with a sound understanding of the underlying clinicopathological mechanisms. This is particularly crucial because serum ferritin level is highly dynamic and not exclusively unidirectional (i.e., decrease or increase); rather, it can change in both directions simultaneously, although it usually increases to a greater extent in the context of critical illness. Anemia of inflammation in critical illness is an excellent example of how such concurrent changes can lead to underperformance of positive acute-phase reactants such as serum ferritin level [98]. The net change in serum ferritin level is an outward manifestation stemming from normal and pathological pathways that warrants further study [99]. Understanding the mechanistic underpinnings allows for better clinical conditions and/or outcome targets.
5. Strengths and limitations
To the best of our knowledge, this is the first systematic scoping review of ferritin levels in critical pediatric illness. We used an a priori defined protocol with specific eligibility criteria sufficient to limit unnecessary information but still allowed comprehensive thematic scoping. However, our study was limited by its lack of access to subscription-based databases, which may have narrowed our search results and unintentionally excluded relevant studies.
In conclusion, despite rapidly growing evidence about the predictive ability of ferritin level, its translation to clinical practice is still hampered by underrepresentation of demographic features, inadequate sample size, and lack of methodological rigor and understanding of underlying pathways. Future directions include increased precision in serum ferritin measurements followed by close attention to the threshold modeling strategy and reporting.
Supplementary material
Supplementary materials: Supplementary materials 1-3 can be found via https://doi.org/10.3345/cep.2022.00654.
Search strategy.
Symbol map showing country of origin, number of studies (color scale), and number of study subjects (circular area and label).
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.