Predictors of high-flow nasal cannula failure in pediatric patients with acute respiratory distress
Article information
Abstract
Background
Heated humidified high-flow nasal cannula (HFNC) has gained popularity recently and is considered a standard respiratory support tool for pediatric patients with acute respiratory distress. However, data are limited on the bedside parameters that can predict HFNC failure in pediatric patients.
Purpose
To evaluate the performance of SpO2/FiO2 (SF) ratio, pediatric respiratory rate-oxygenation (pROX) index, and clinical respiratory score (CRS), for predicting the HFNC outcomes.
Methods
This prospective observational study included 1- month to 15-year-old patients with acute respiratory distress who required HFNC support. The HFNC setting, vital signs, CRS, and treatment outcomes were recorded. Data were analyzed to determine the predictors of HFNC failure.
Results
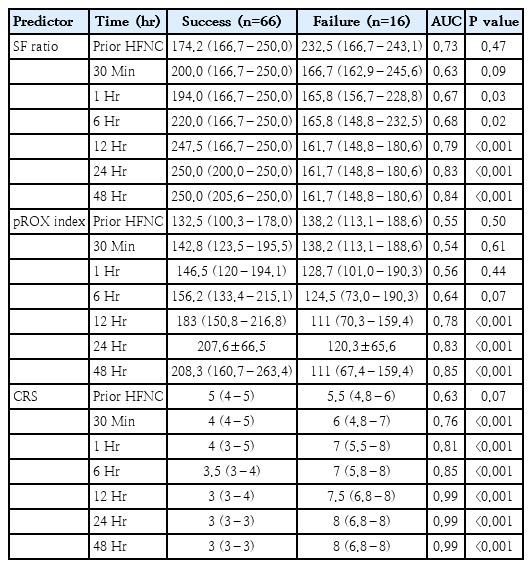
Eighty-two children participated in the study, 16 of whom (19.5%) did not respond to HFNC treatment (failure group). Pneumonia was the main reason for intubation (62.5%). Predictors of HFNC failure at 12 hours were: SF index ≤166 (sensitivity, 62.5%; specificity, 87.8%; area under the curve [AUC], 0.75), pROX index <132 (sensitivity, 68.7%; specificity, 84.8%; AUC, 0.77), and CRS ≥6 (sensitivity, 87.5%; specificity, 96.9%; AUC, 0.92).
Conclusion
The CRS was the most accurate predictor of HFNC failure in pediatric patients. A CRS ≥ 6 at 12 hours after HFNC initiation and pROX, a newly modified parameter, are helpful indicators of HFNC failure.
Key message
SpO2/FiO2 ratio ≤166, pediatric respiratory rate-oxygenation index <132, and clinical respiratory score ≥6 at 12 hours after high-flow nasal cannula (HFNC) initiation were useful bedside predictors for HFNC failure in pediatric patients.
Graphical abstract. HFNC, high-flow nasal cannula; SF, oxygen saturation to fraction of inspired oxygen ratio; AUC, area under the curve; pROX, pediatric respiratory rate-oxygenation.
Introduction
Recently, heated humidified high-flow nasal cannula (HFNC) has gained popularity and is used as standard respiratory support in pediatric patients with acute respiratory distress. Several studies have shown the benefits of HFNC, such as good outcomes, improvement in physiologic parameters, and decreased intubation rates [1-3]. Furthermore, most pediatric patients tolerate HFNC better than they tolerate other types of respiratory support. The incidence of HFNC treatment failure depends on the patient characteristics and indication of HFNC use and varies from 10%– 20% [3-5]. Understanding and predicting the outcomes of HFNC treatment are crucial for improving bedside patient care and monitoring. Delayed intubation in patients that require HFNC may lead to poor outcomes [6]. Previous studies on the predictors of HFNC outcomes in the pediatric population mostly used the Pediatric Early Warning System (PEWS) respiratory score [7] and vital signs as the risk factors for treatment failure. However, the common parameters investigated in the previous studies depended on the age group, and their study population might not be representative of all pediatric patients. Most studies have shown that a worsening trend of vital signs along with the respiratory score was associated with poor outcomes and treatment failure of HFNC. However, there are no data on the performance of these parameters, including the cutoff values. Roca et al. [8] evaluated the utility of SpO2/FiO2 (SF) ratio in pneumonia patients with hypoxemic respiratory failure and described the respiratory rate-oxygenation (ROX) index, which is the ratio of SF to the respiratory rate (RR). A subsequent study was conducted to validate the ROX index [9] in adults with pneumonia requiring HFNC treatment. Resultantly, a ROX index of more than 4.88 at 12 hours after HFNC initiation could predict a negative outcome (hazard ratio, 0.291; 95% confidence interval [CI], 0.161–0.524; P<0.001). Unfortunately, there is no study on the usefulness of the ROX index in pediatric patients. Additionally, the pediatric population has a varied range of RRs and vital signs. The clinical respiratory score (CRS) (Supplementary Table 1) is one of the many validated respiratory scores to classify patient severity [10]. Bedside assessment of CRS can be performed by simple observation in all age groups in the pediatric population. Therefore, the development of bedside indexes for prediction of HFNC outcomes could guide clinical decision making. Hence, we conducted this study to evaluate the performance of bedside parameters, such as the SF ratio, pediatric ROX index, and CRS, for predicting the HFNC outcomes.
Methods
This study was a prospective observational study conducted at a tertiary university hospital from July 2019 to February 2020. This study was approved by Human Research Ethic Committee of Faculty Medicince, Prince of Songkla University (REC 62- 067-1-1), and written informed consent was obtained from the patients’ parents/guardians before inclusion. Patients aged 1 month to 15 years with respiratory distress of any etiology and who received HFNC were included in the study. Patients with congenital cyanotic heart diseases were excluded from this study due to the low SpO2 in this population. The primary objective was to determine the predictors of HFNC treatment failure. The secondary objective was to identify the cutoff value of each parameter. Data on the patient’s characteristics, indication for HFNC usage, HFNC setting, parameters after HFNC treatment, and the outcomes of HFNC treatment were recorded. HFNC failure was defined as the need for invasive mechanical ventilation within 48 hours after HFNC initiation.
1. Predictor indices
The ROX index was used to predict the HFNC outcomes based on a previous study in adults [8]. ROX index was defined as the ratio of SF to the RR. However, the RR in the pediatric population varies depending on the age group. Therefore, we used respiratory rate standard (RRSD), which was defined as the ratio of the RR of the patient to the normal RR of that age group (40 in children aged less than 1 year, 30 in 1- to 5-year-olds, and 20 in more than 5-year-olds). The newly developed pediatric ROX (pROX) index was defined as the ratio of SF to RRSD. The other predictors analyzed were the CRS, vital signs, and SF ratio. CRS is used in standard patient care to classify the severity of respiratory distress into mild, moderate, or severe. Inter-rater reliability of CRS was evaluated to ensure standard assessment of all patients, and the results indicated 75% agreement.
All parameters were recorded before starting HFNC treatment, then at 30 minutes, 1, 2, 6, 12, 24, and 48 hours after HFNC treatment.
2. Patient management
All patients received standard care during HFNC treatment, including the standard HFNC circuit with the humidifier setting at 37℃. The initial flow was started with at least 1 LPM/kg and FiO2 of 1.0. Subsequently, the FiO2 was adjusted according to the patient’s target SpO2 (higher than 94%). The amount of flow was adjusted according to the presence of continuous flow in both basal lung areas based on the attending physician’s examination. The attending physician modulated the amount of flow as well as the FiO2 according to the clinical condition of the patient to achieve SpO2 >94%. As per the institutional standard protocol, the indications for HFNC were patients who had moderate-tosevere respiratory distress or mild respiratory distress which did not respond to conventional oxygen therapy. The indications for intubation were clinical signs of respiratory failure, including persistent hypoxemia without response to HFNC treatment, decreased level of consciousness (Glasgow coma score <12), and significant cardiovascular instability (Supplementary Fig. 1)
3. Statistical analysis
The estimation of the sample size was based on the expected sensitivity and specificity of the predictive index, which was 80 %, and the estimated incidence of HFNC failure, which was about 20%. The final sample size was 80. Continuous variables were presented as means with standard deviations or medians with interquartile ranges. Categorical variables were expressed as frequencies (percentages). Comparisons between continuous variables were made using the Student t test or Mann-Whitney U test. The differences between the categorical variables were evaluated with the chi-square or Fisher exact test. To determine the accuracy of the predictors of HFNC failure, receiver operating characteristic curve was constructed and the area under the curve (AUC) was calculated. This study defined 0.7 as an acceptable AUC for prediction of HFNC failure. The cutoff value of each predictor was evaluated by the most appropriate AUC. Kaplan-Meier curves were used to evaluate the probability of HFNC failure for each cutoff value. A P value less than 0.05 was considered statistically significant. The analysis was performed with R ver. 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 82 patients undergoing HFNC treatment participated in this study. There were 16 patients with failed HFNC treatment who required invasive mechanical ventilation; the HFNC failure rate was 19.5%. Pneumonia was the most common diagnosis among the HFNC failure group (62.5%). The baseline characteristics and patient outcomes are described in Table 1. Patients who responded to HFNC treatment had a longer duration of HFNC treatment (86.9±191.2 hours vs. 10.3±9.84 hours, P<0.001).
1. Predictor analysis
This study focused on the CRS, SF ratio, and pROX index as predictors of HFNC failure. There was no difference among the predictor variables in both the HFNC treatment success and failure groups (Table 2). In the HFNC failure group, the SF ratio and pROX index decreased over time, while the CRS increased over time (Fig. 1). The SF ratio at 1 hour after HFNC treatment was significantly lower in the HFNC failure group (165.8 [156.7–228.8] vs. 194 [166.7–250], P=0.03), whilst the pROX index significantly decreased in the HFNC failure group from 12 hours after HFNC initiation (111 [70.3–159.4] vs. 183 [150.8–216.8], P<0.001). The CRS was significantly higher in the HFNC failure group at 30 minutes after HFNC initiation (6 [4.8–7] vs. 4 [4–5], P<0.001).

Comparison of each index value overtime time period between success of HFNC treatment (blue) and failure of HFNC treatment (red). (A) SF ratio. (B) pROX index. (C) Clinical respiratory score. In the HFNC failure group, the SF ratio and pROX index decreased over time, while the CRS increased over time. HFNC, high-flow nasal cannula; SF, oxygen saturation to fraction of inspired oxygen ratio; pROX, pediatric respiratory rate-oxygenation index; CRS, clinical respiratory score.
AUC was used to determine the best cutoff values of the predictive factors. An SF ratio ≤166 at 12 hours after HFNC initiation predicted HFNC failure with a sensitivity of 63% and specificity of 88%. A pROX index<132 at 12 hours after HFNC initiation predicted HFNC failure with a sensitivity and specificity of 69% and 85%, respectively. A CRS ≥6 at 12 hours after HFNC initiation predicted HFNC failure with a sensitivity of 88% and specificity of 97% (Table 3). The Kaplan-Meier curves of the probability of HFNC failure at the cutoff value of each predictor are demonstrated in Fig. 2. A comparison of the performance of each index is shown in Fig. 3. In this study, CRS ≥6 at 12 hours after HFNC initiation was the best predictor of HFNC failure with an AUC of 0.92. The subgroup analysis was performed for patients with pneumonia diagnoses. The performance of all diagnostic tests was approximated to the whole population (Supplementary Table 2).

Diagnostic accuracy of predictive indexes for highflow nasal cannula failure at 12 hours posttreatment
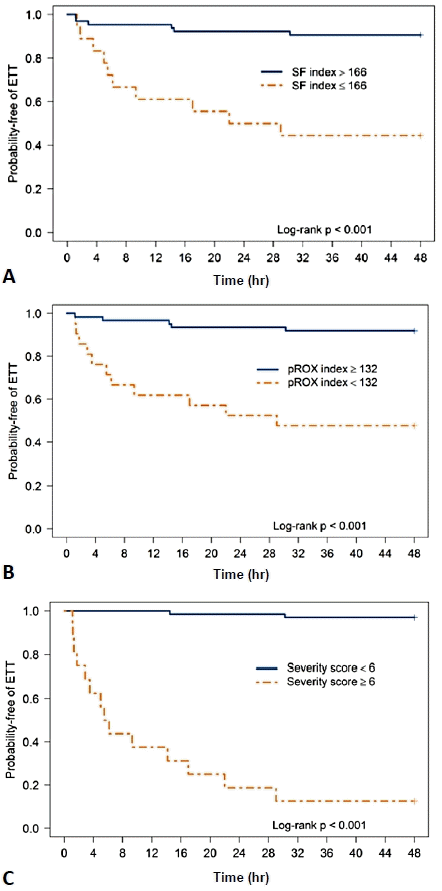
The Kaplan-Meier curves of probability of HFNC failure at of the cutoff value of each index was demonstrated. (A) Cutoff value of SF ratio≤166. (B) Cutoff value of pROX index<132. (C) Cutoff value of clinical respiratory score≥6. A blue line indicated the survival curve of the patient group with higher levels than cutoff point. An orange line the survival curve of the patient group with lower levels than cutoff point. The P values are determined by log-rank tests. HFNC, high-flow nasal cannula; SF, oxygen saturation to fraction of inspired oxygen ratio; pROX, pediatric respiratory rate-oxygenation index; ETT, .

The area under the curve (AUC) of predictive indexes cut point; SF>166 (red), pROX>132 (green), and CRS≥6 (blue) for HFNC failure at 12 hours after treatment. The CRS≥6 at 12 hours after HFNC initiation was the best predictor of HFNC failure with an AUC of 0.92. SF index≤166 and pROX<132 at 12 hours after HFNC initiation had AUC of 0.75 and 0.77 respectively. HFNC, high-flow nasal cannula; SF, oxygen saturation to fraction of inspired oxygen ratio; pROX, pediatric respiratory rate-oxygenation index; CRS, clinical respiratory score.
Discussion
HFNC is considered a standard respiratory support tool in pediatric patients with respiratory distress. The challenge for physicians caring for patients on HFNC is to avoid delayed intubation during the transition from HFNC to other respiratory support tools. This study demonstrated a HFNC failure rate of 19.5%, which was similar to that in previous reports [4,5,7]. Identifying patients at risk of HFNC failure is crucial for optimizing the patient outcomes. Previous studies have used the trend of vital signs (RR, heart rate) and arterial blood gas results (PaCO2 or PaO2/FiO2) as surrogate predictors. In addition, the respiratory items (RR, SpO2, chest retractions) in PEWS were also used to predicted the HFNC outcome [7]. However, most of these studies did not determine the cutoff values or describe the accuracy of these parameters. Moreover, vital signs may be affected by other conditions, and arterial blood gas measurement is also considered an invasive test, especially in pediatric patients. In this study, we used less invasive parameters, which can be easily assessed at the bedside, including the SF ratio, pROX index, and CRS.
Since SpO2 is a standard monitoring parameter in patients with respiratory problems, the SF ratio is commonly used to identify the severity of respiratory illness and also as a diagnostic criterion for pedatric acute respiratory distress syndrome [11]. The SF ratio had a strong correlation with the PaO2/FiO2 ratio, but the SF ratio was considered easily accessible and required less invasive monitoring compared to the PF ratio [12,13]. Kamit et al. [5] found that an SF ratio>200 at 1 hour after HFNC initiation predicted the success of HFNC therapy (odds ratio [OR], 8.034; 95% CI, 2.981–21.657; P<0.001). Er et al. [4] revealed that an SF ratio<195 at 1 hour after HFNC treatment was associated with treatment failure. These 2 retrospective studies demonstrated the association between a lower SF ratio and treatment failure. However, they could not identify the accuracy of the SF ratio nor its cutoff value for outcome prediction. Kim et al. [14] found that SF ratio<230 at initiation of HFNC had an AUC of 0.75 in terms of prediction of HFNC failure, which is better than that of the PF ratio. The univariate analysis revealed that SF ratio<200 at 2 hours was associated with HFNC failure in acute hypoxic respiratory failure. In the present study, an SF ratio≤166 at 12 hours after HFNC initiation predicted HFNC failure with a sensitivity of 63%, specificity of 89%, negative predictive value (NPV) of 91%, positive predictive value (PPV) of 56% and an AUC of 0.75.
The pROX index is a novel parameter developed in this study. It is a modification of the adult ROX index, which uses the normal RR in adults (RR=20). RRSD was used instead of RR in the pROX index calculation. RRSD is the ratio of the patient’s RR to the normal RR for the age group. pROX is also easily calculated and can be used as bedside parameter. In a similar study, Yildizdas et al. [15] calculated the ROX index in pediatric patients by using the respiratory z score instead of the RR. They found that an ROX index above 66.7 at 24 hours after HFNC had 86% sensitivity, 79% specificity, 23.1% PPV, and 98.8% NPV. A previous retrospective study by Krachman et al. [16] used machine learning algorithms of ROX index to predict flow rate escalation. In the present study, we used the normal RR for age to adjust the ROX index. pROX index<132 in this study had good discriminating power for prediction of HFNC failure with 69% sensitivity, 85% specificity, 52% PPV, and 92% NPV. To our knowledge, there is currently no standard definition of pediatric ROX. The only study that defined pediatric ROX was the study by Yildizdas et al. [15]; however, they used the respiratory z score, which is relatively complicated to apply at the bedside. In the present study, we used the normal RR for each pediatric age group, which can be easily calculated.
The CRS is used to classify patients according to the severity of respiratory distress, which can be helpful in treating patients in the emergency setting [10]. Furthermore, CRS was validated in a previous study and can help identify respiratory distress severity in all pediatric age spans [17]. Our institution used the CRS to classify patients with acute respiratory distress into mild, moderate, and severe. To our knowledge, there has been no study on the use of CRS for HFNC outcome prediction. Although the CRS comprises many items and subjective, it is easy to use. The inter-rater reliability of CRS was 75%. The present study demonstrated that CRS≥6 at 12 hours after HFNC treatment had the most discriminating power to predict HFNC failure with an AUC of 0.92. Although CRS was identified as the most accurate index for predicting HFNC failure in terms of bedside application and generalizability, it still requires additional studies for validation.
The main shortcoming of the previous studies [4,5,7,18] was the study design, as most of these studies were retrospective. Patients who failed HFNC treatment were more unwell than patients who exhibited successful outcomes. Consequently, the previous studies showed significantly different parameters in the early period (first to 6 hours) after HFNC initiation. In the present study, all patients had mild-to-moderate respiratory distress at the commencement of HFNC therapy. Patients with severe respiratory distress as well as respiratory failure were not included in the study because this population was too unwell to receive HFNC therapy. This may explain the results of most predictors becoming significant at 6 and 12 hours after HFNC initiation in our study.
This study had some limitations. First, the indications for intubation did not include the laboratory results, such as the arterial blood gas. The application of general pediatric intubation criteria was more pragmatic and generalizable. Second, our study population was small and heterogeneous cohort. However, our sample size had sufficient power to demonstrate the accuracy of the identified predictors. The major indicator for HFNC treatment in this study was pneumonia and the performance of each index was close to that of the overall population. Generalization of these results should be made judiciously. Additional studies with larger populations are required for the replication and validation of the predictive score (SF, pROX, and CRS) in the future.
This study showed that the SF ratio, pROX index, and CRS were useful bedside predictors for HFNC failure in pediatric patients. CRS was the most powerful predictor of HFNC failure. CRS ≥6 at 12 hours after HFNC initiation had a PPV of 96.8% (AUC, 0.92). Patients who had an SF ratio>166 and a pROX index score≥132 were less likely to need intubation with an NPV of 90.6% and 91.8%, respectively.
Supplementary materials
Supplementary Tables 1-2 and Fig. 1 can be found via https://doi.org/10.3345/cep.2022.00766.
Clinical respiratory score[10]
Diagnostic accuracy of predictive indexes for HFNC failure at 12 hours after treatment in patient with diagnosis of pneumonia
Diagnostic accuracy of predictive indexes for HFNC failure at 12 hours after treatment in patient with diagnosis of pneumonia
Notes
Conflicts of interest
All the authors declared no conflict of interest.
Funding
This study was funded by Faculty of Medicine, Prince of Songkla University.
Author Contribution
KS, BT; Investigation: KS, BT; Methodology: KS, BT; Project Administration: KS, BT, K, PP, WA; Writing – Original Draft: KS, BT, KR, PP, WA; Writing – Review & Editing: KS, BT, KR, PP, WA