Global varicella vaccination programs
Article information
Abstract
Varicella (chickenpox) is an infectious disease caused by the highly contagious varicella zoster virus with a secondary attack rate greater than 90%. From this perspective, we aimed to establish the basis for a national varicella vaccine policy by reviewing vaccination programs and policies of countries that have introduced universal varicella vaccinations. As a result of the spread of varicella, an increasing number of countries are providing 2-dose vaccinations and universally expanding their use. In practice, the efficacy and effectiveness of vaccination differ among vaccines and vaccination programs. Optimized vaccination strategies based on each country’s local epidemiology and health resources are required. Accordingly, it is necessary to evaluate the effectiveness of varicella vaccines in different settings. Given the short-term and fragmented vaccine effectiveness evaluation in Korea, it is necessary to evaluate its effectiveness at the national level and determine its schedule based on the evidence generated through these studies.
Key message
It is important to evaluate its effectiveness at the national level and to determine the varicella vaccine schedule based on the evidence generated through the studies.
Graphical abstract
Introduction
Varicella is a communicable disease caused by the highly contagious varicella zoster virus (VZV), with a secondary incidence of >90% in susceptible populations [1,2]. Varicella is generally considered a mild disease in healthy children, with symptoms such as an itchy rash, malaise, pruritus, and fever for 2–3 days. However, VZV becomes latent in the neural ganglia after the primary infection. The incubation period typically lasts a lifetime, and the virus can be reactivated as herpes zoster when one’s immunity declines [3,4].
Active immunization is an important preventive intervention for varicella, vaccines for which are reportedly effective at reducing its worldwide incidence and disease burden [5]. The World Health Organization (WHO) recommends introducing universal varicella vaccinations in countries in which varicella provides an important burden of disease under conditions that can ensure reaching and sustaining a high vaccine coverage of more than 80% [6].
For effective prevention of varicella, it is important to both maintain high vaccine coverage and have a strategic plan against vaccine failure. Vaccine failure can be classified as primary, referring to inadequate primary immune mounting, and secondary, generally caused by waning immunity over time [7]. A single-dose varicella vaccine may effectively reduce disease severity, but is prone to breakthrough infections [8-12]. A 2-dose varicella vaccine reduces the disease severity and the incidence of breakthrough cases [13-16]. Since the introduction of the varicella vaccine as a NIP in 2005, Korea has achieved a high vaccine coverage of 96.3% (as of 2019) [17]; however, breakthrough cases are still reported [18,19].
In 1995, the United States was the first country to adopt a 1-dose varicella vaccine as a universal program. Since then, countries that introduced the varicella vaccine as a universal program have evaluated its effectiveness through a study of disease burden [3,5,20-23]. However, some countries still have no universal varicella vaccine program for infants and selectively vaccinate only high-risk groups or healthcare workers [24]. This is because healthy children often develop mild symptoms when infected with varicella; hence, the urgency lags behind that of other new vaccines, including those for the human papilloma virus, rotavirus, and Streptococcus pneumoniae [24,25].
The consequences of the age shift of varicella cases to older age groups are also pushing back a universal varicella vaccination on the priority list. The reduction in varicella incidence in children reduces the chance of natural immune boosting in the elderly population, resulting in an increase in the incidence of herpes zoster infection. Moreover, the age shift in varicella infection may lead to a more severe disease [24,26]. Other factors such as cost-effectiveness of the vaccination program also influence public health priorities. Considering the health investment trade-offs, a country has 3 options to choose from regarding the national immunization schedule: no adoption, a 1-dose vaccination regimen to alleviate disease severity, or a 2-dose vaccination regimen to ultimately eradicate VZV.
Here we assess the rationale for vaccination programs to derive the necessary basis for domestic varicella vaccine policy by reviewing vaccination programs in different countries and describing their policy of introducing a universal varicella vaccination program.
We conducted a nonsystematic review to identify the countries in which the varicella vaccine was provided as a universal vaccination program. Countries providing the varicella vaccine as a universal vaccination program were investigated through the websites of the WHO and the European Center for Disease Prevention and Control. This comprehensive literature review aimed to obtain a complete record of all the available literature on varicella vaccination programs in different countries using the following PubMed search terms: country + varicella, vaccine, country + vaccination, country + schedule, country + vaccination program, country + universal vaccination, country + vaccine strain, and country + coverage. The countries were grouped into 6 regions according to WHO groupings: Western Pacific, Europe, America, Eastern Mediterranean, Africa, and Southeast Asia.
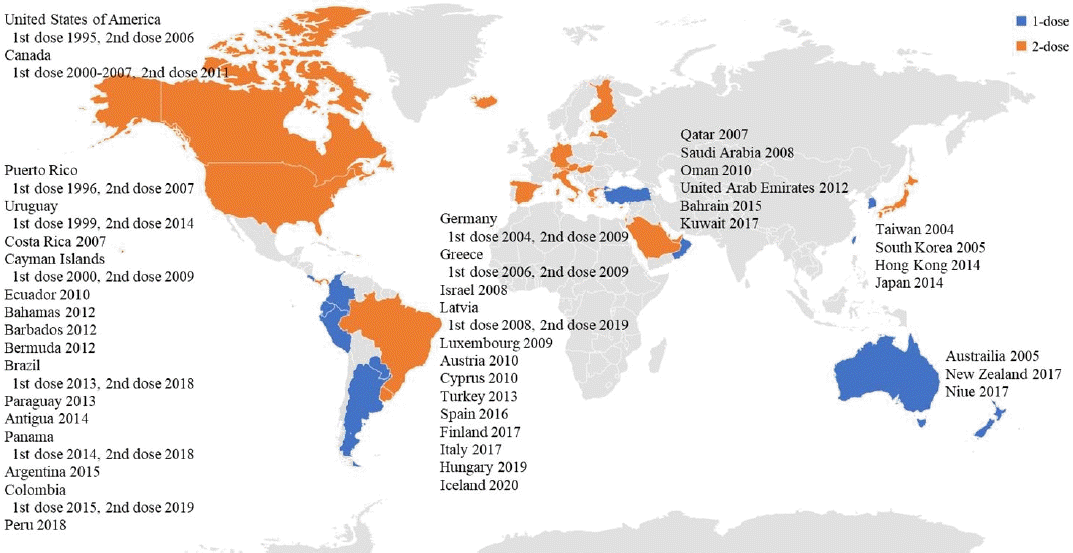
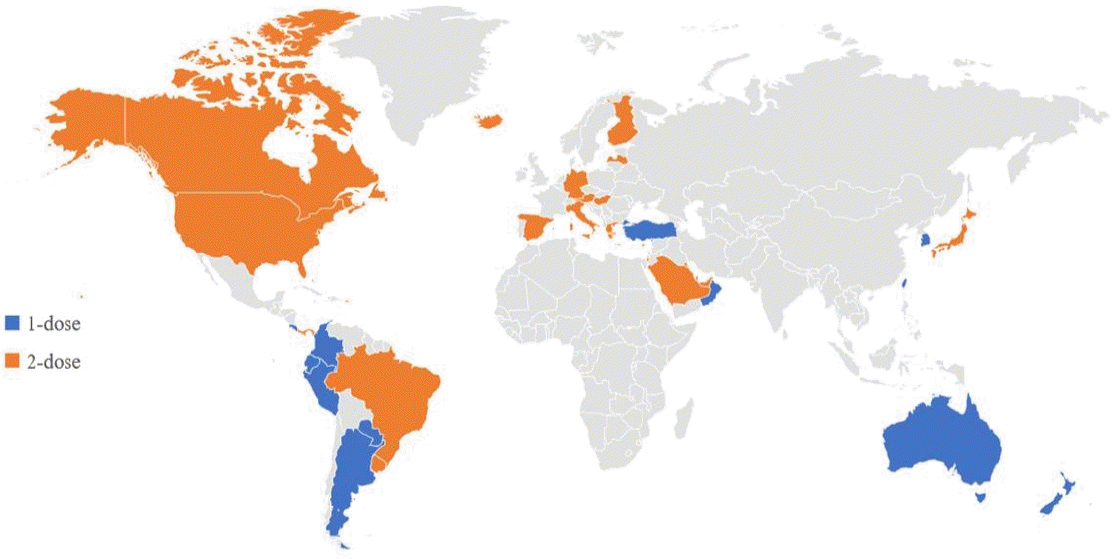
The varicella vaccine is a live attenuated vaccine. The Oka vaccine was developed in Japan in 1974, and the MAV vaccine, another live attenuated varicella vaccine, was developed in Korea in 1993 [27,28]. Except for 1 type of varicella vaccine based on the MAV strain, which is licensed in Korea, the Oka strain is used globally for varicella vaccines [29]. Currently licensed live attenuated vaccines include the monovalent varicella vaccine and measles, mumps, rubella and varicella (MMRV) vaccine, a tetravalent vaccine (Table 1). As of the first half of 2021, 44 countries have introduced the varicella vaccine as a universal vaccination for infants. However, the vaccination schedules vary in that some countries use a single dose, whereas other countries use a second dose (Table 2, Fig. 1). Overall, 28 countries use a universal 2-dose varicella vaccine regimen for infants; mandatory vaccinations are provided in all countries except Austria and Cyprus.
Varicella vaccination status by region
1. Western Pacific region
Only a few countries in the Western Pacific region have adopted a universal varicella vaccine regimen. Taiwan was the first country to introduce the varicella vaccine in 2004, followed by Australia and Korea in 2005. Among the countries that introduced a universal varicella vaccine, all but Hong Kong and Japan started a 1-dose vaccination; 17 years later, a high vaccination rate (>90%) was confirmed in Australia, Korea, and Taiwan [17,30,31]. In Japan, where the first varicella vaccine (Oka) was developed, a universal vaccination for infants was introduced in 2014, but the vaccination rate was low (about 40%) [32]. As of 2021, the varicella vaccine has been used in 7 Western Pacific countries, and the MMRV vaccine combined with the MMR vaccine has only been used in Hong Kong as a 2-dose regimen.
In Korea, the varicella vaccine was introduced in the private market in 1988, and it has been recommended as an option for high-risk groups and healthy children [7]. Since 2005, varicella vaccinations have been recommended as part of the national immunization program for all children aged 12–15 months. The varicella vaccine was an imported product until 1993 in Korea, after which time several varicella vaccines were developed by domestic companies. However, imported vaccines were also used. The varicella vaccines developed in Korea include Okabased vaccines such as Suduvaccine (CJ Cheil, Seoul, Korea), LG Suduvaccine (LG Chemical, Seoul, Korea), and the MAV-based Suduvax (GC Green Cross, Yongin, Korea) [7]. The varicella vaccines on the market in Korea as of the first half of 2021 are Oka-based, namely Vari-L (2,000 plague-forming units [PFU]; Changchun Institute of Biological Products, Jilin, China), SKY varicella (2,400 PFU; SK Bioscience, Seongnam, Korea), the MAV-based Suduvax (1,400 PFU; Green Cross), and varicella vaccine (3,800 PFU; Green Cross) (Table 1). Priorix-Tetra (GSK, London, UK), an MMRV vaccine, was approved by the Ministry of Food and Drug Safety in 2013, but it is not currently on the market.
2. European region
In Europe, varicella vaccines are used in 28 countries, of which 16 use MMRV vaccines [3]. As of 2021, varicella vaccination is recommended by the National Immunization Program (NIP) in 14 countries, 12 of which are conducting a universal vaccination program.
The first countries in Europe to adopt the varicella vaccine as a 1-dose vaccination schedule were Germany in 2004 and Greece in 2006; both countries began to administer a universal 2-dose schedule. In Germany, a 2-dose vaccination policy was adopted because of persistent community and individual-level breakthrough infections despite the introduction of a one-dose vaccination regimen [33]. In Greece, although the effectiveness of a 1-dose vaccination has not been evaluated because varicella is not a notifiable disease, a 2-dose vaccination regimen was introduced to enhance immunity [34].
Italy and Spain introduced universal vaccination programs by region and then expanded them country-wide. In Italy, Sicily first introduced a universal varicella vaccine regimen in 2003; Veneto introduced a 2-dose vaccination regimen in 2005 for children aged 14 months and 6 years; and Toscana started a 1-dose vaccination program in 2008 [35]. Since then, through its National Plan for Vaccination, Italy implemented a mandatory universal vaccination for infants nationwide in 2017 [36].
Spain introduced a 1-dose vaccination program for children at 15 months of age, and a catch-up vaccination was provided in November 2006 for children aged 10 years in Madrid and for those susceptible due to missed vaccination. Other areas of Spain implemented a different regimen in 2007, namely a 2-dose varicella vaccination for children aged 15 months and 3 years in Navarre. In Ceuta and Melilla, a 2-dose vaccination program was introduced in 2009 for children at 18 and 24 months and 15 and 24 months, respectively. The rest of the region introduced catch-up vaccinations for high-risk groups and children under 12 years of age [3,35]. Eventually, a universal varicella vaccination program for the whole country was adopted as a national immunization policy in 2016, and a 2-dose vaccination program was provided for children aged 15 months and 3 years [3].
Luxembourg began providing varicella vaccinations for high-risk groups in 2003 and introduced a 2-dose universal vaccination program for children aged 12 months and 15–23 months in 2009 [35]. It is the only European country that provides 2 doses of the MMRV vaccine.
Other countries, such as the United Kingdom (UK), have not introduced the varicella vaccine into their national immunization schedules because they consider varicella a mild disease and age shift as a burden of disease for the elderly including more severe complications and an increase in herpes zoster infection rates [25,37]. Moreover, some countries such as the UK and France have found that it is not cost-effective to adopt a universal varicella vaccination regimen [24].
3. American region
The United States was the first country to introduce a universal varicella vaccine regimen. In 1995, a 1-dose vaccination program was introduced at 12–15 months of age, while in 2006, the recommendation of a second dose for children 4–6 years of age was adopted. It has also been recommended that children, adolescents, and adults who were previously vaccinated receive a second dose [25].
Canada approved the varicella vaccine in December 1998; from 2000 to 2007, 13 regions introduced a universal vaccination program that provided a single dose at the age of 12–15 months [38]. However, breakthrough infections occur, and some patients are severely affected. This suggests that in a population vaccinated with only a single dose, immunity may wane over time, resulting in an increasing incidence among adolescents and adults, who may develop severe varicella symptoms and be prone to complications. In 2010, the National Advisory Committee on Immunization recommended a 2-dose varicella vaccine regimen [39]. As a result, a universal 2-dose vaccination program was implemented in 2011.
In 2016, the Latin American Society of Pediatric Infected Disorders recommended a national mandatory 2-dose varicella vaccine regimen in Latin America and the Caribbean. As of 2021, it has become a universal vaccination regimen in 15 countries, of which 7 provide 2 doses [40]. In other countries in the region, universal vaccination has not been introduced, but vaccinations are administered to high-risk groups. In Mexico, vaccinations are recommended for children in daycare facilities, immunodeficient patients, childhood cancer patients, and susceptible medical workers [41].
4. Eastern Mediterranean, African, and Southeast Asian regions
Six countries in the Middle East use a universal varicella vaccination program, and in all but Oman, a 2-dose schedule is implemented. Both Qatar and the United Arab Emirates have vaccine coverage rates exceeding 90% [42,43].
Three Southeast Asian countries (India, Indonesia, and Thailand) recommend varicella vaccination but have not added it to their NIP [44].
None of the other Southeast Asian or African countries have introduced a universal varicella vaccine regimen because of limited resources and the need to prioritize other vaccinated infectious diseases that create a much higher burden of disease on public health [45].
Varicella vaccine utilization in Korea
In Korea, a varicella vaccine was first licensed and distributed via the private market in 1988 [46]. Varicella became nationally notifiable in July 2005, when 1 dose of varicella vaccine was introduced to the NIP and recommended for children aged 12–15 months. Since it was designated a group 2 infectious disease in 2005, it has been included in the Korea Disease Control and Prevention Agency’s (KDCA) acute infectious disease annual monitoring report. Medical doctors, Oriental medical doctors, and heads of public health centers or commanders of the armed forces are required to immediately report a confirmed or probable case through a web-based reporting system.
According to the KDCA, 11,027 cases were reported in 2006 versus 20,284 in 2007, 44,450 in 2014, and 96,467 in 2018 [47]. Reported varicella cases slightly declined to 82,868 in 2019 and further decreased to 31,430 in 2020 due to coronavirus disease 2019 [48]. However, the Health Insurance Review and Assessment Service data from the National Health Insurance Service showed that the number of claims decreased during the period, with 194,283 cases in 2011, 101,861 cases in 2014, 86,465 cases in 2018, and 39,127 cases in 2020 [49]. Although these trends in varicella rates were inconsistent and involved their own bias, multiple studies assessing the effectiveness of varicella vaccine in Korea concluded insignificance of the varicella vaccine [19,50].
The number of vaccines approved for shipment in Korea ranged from 1,561,578 dozen (2012) to 4,695,282 dozen (2017) per year from 2011 to 2019, which is at least 3 to 10 times more than the 1-year birth cohort (Fig. 2). Meanwhile, the first vaccination rates for varicella vaccine exceeded 97% in 2015–2019, and it is assumed that the overshipped vaccines were used as secondary or catch-up doses because they were not counted in the statistics (Fig. 2).
Annual varicella vaccine distribution and vaccination coverage, South Korea. Distribution is the number of vaccine doses distributed per year, while coverage rate is percentages of age-specific cohorts receiving vaccinations.
Varicella is an important vaccine-preventable disease causing a significant burden not only in childhood but throughout the lifespan, resulting in shingles in the elderly population. The effectiveness of vaccination varies from 83% for the single dose to 95% for the 2 doses, which clearly shows the association between the number of doses and public health benefits [6]. We found an increased number of countries that included a 2-dose varicella vaccine regimen in the setting of expanding their program. The number of doses distributed annually does not accurately reflect the number of doses administered to children; therefore, our data require careful interpretation. Because the efficacy and effectiveness of vaccines and programs may vary among settings, vaccination strategies should be optimized for each country in the context of local epidemiology trends and health resources. Accordingly, the effectiveness of varicella vaccines requires separate evaluation under different conditions.
Conclusion
Real-world data on varicella vaccine effectiveness in Korea are currently limited. Previous studies demonstrated significant waning of immunity in those who received a 1-dose varicella vaccine [19,50]. For those who received 2-dose varicella vaccines, immunity waning has not yet been determined in a public program supporting a 1-dose vaccine strategy. Therefore, it is important to evaluate the effectiveness of the varicella vaccination program at the national level over a longer observation period to develop a data-driven policy to guide vaccination programs.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: CYJ , KYK; Data curation: LYH, LJ, KE, LJY; Formal analysis: LYH, HK; Funding acquisition: KYK; Methodology: LYH, CYJ, KYK; Project administration: Lee J, Kim E, LJY; Visualization: LYH, CYJ; Writing - original draft: LYH, CYJ, KYK; Writing - review & editing: LYH, CYJ, KYK