Clinical considerations and practical issues of allergic diseases in COVID-19 era
Article information
Key message
The risk of sudden acute respiratory syndrome coronavirus 2 infection and severe coronavirus disease 2019 (COVID-19) outcomes is not elevated in patients with the type 2 phenotype and well-controlled asthma. Inhaled corticosteroids, intranasal corticosteroids, and topical steroids can be safely used in COVID-19 patients. Biologics can be safely used by patients with allergic diseases without concern about antibody responses.
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection outbreak, burdened healthcare system capacities. It also reshaped doctor–patient interactions, favoring a reduction in face-to-face visits. Accordingly, it has created many questions about the optimal and safest way to manage patients with allergic diseases, traditionally considered face-to-face evaluations, including physical examinations, pulmonary function tests, and allergic laboratory tests. However, face-to-face patient interactions have been limited by the pandemic. Therefore, it is necessary to prioritize management plans for patients with allergic diseases based on scientific rationale. Individualized plans are needed because the associations between COVID-19 and the clinical outcomes of allergic diseases are affected by diverse factors. The present editorial summarizes the relationship between allergic diseases and COVID-19-related outcomes and reviews the clinical considerations and practical issues of managing allergic diseases in the COVID-19 pandemic era (Fig. 1).
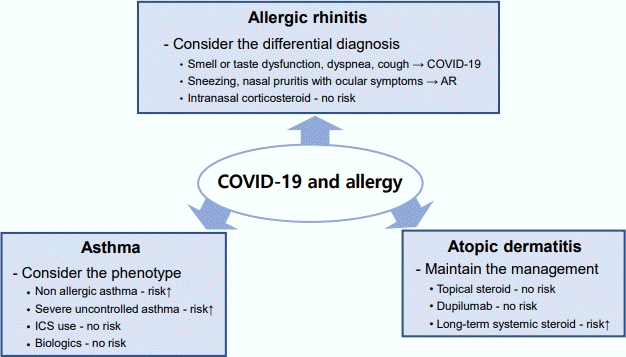
Clinical considerations of allergic diseases in the coronavirus disease 2019 (COVID-19) era. In asthma, phenotype should be considered a risk factor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and severe COVID-19 outcomes are elevated in patients with nonallergic asthma and severe uncontrolled asthma. Differential diagnosis is important in allergic rhinitis (AR) because AR shares several similarities with COVID-19. Smell or taste dysfunction is a predominant symptom of COVID-19 compared to AR. Management strategies, including topical steroids and dupilumab, can be maintained in patients with atopic dermatitis (AD) without increasing the risk of SARS-CoV-2 infection. ICS, inhaled corticosteroid.
Differences between children and adults should be considered when formulating treatment strategies for allergic diseases. SARS-CoV-2 infections in children have a milder clinical course with less severe morbidity and mortality rates than in adults [1,2], whereas the prevalence of allergic diseases is higher in children than in adults. Several studies have demonstrated that the key host proteins involved in SARS-CoV-2 cellular entry, including angiotensin-converting enzyme 2 (ACE2), transmembrane protease serine subtype 2, and furin protein, may contribute to the differing COVID-19 prognosis of children versus adults [3]. Expression levels of ACE2, the critical initial step for SARS-CoV-2 spike protein binding, are inversely related to type 2 cytokine levels. A study of 3 cohorts of children and adults showed that allergic sensitization was inversely related to ACE2 expression in the nasal epithelium regardless of the presence of asthma in adults, and the degree of allergic sensitization was inversely correlated with ACE2 expression levels in children with asthma [4]. In addition, the mild symptoms and decreased susceptibility to COVID-19 of children versus adults can be explained by the increase in ACE2 expression in the nasal epithelium with age [5].
Corticosteroids, the cornerstone of treatment for allergic diseases, have strong immunosuppressive and anti-inflammatory effects that regulate fundamental processes in the human body and control cellular functions [6]. Their use can mitigate the risk of acute respiratory distress syndrome in COVID-19 and inhibit the development of cytokine storm and multi-organ damage [7]. However, their long-term use in patients with allergic diseases may reduce viral clearance. Therefore, the risk-benefit ratio of corticosteroid use should be assessed in patients with allergic diseases.
Accumulating evidence suggests that the asthma phenotype and endotype may affect COVID-19 outcomes. Several studies have shown that severe uncontrolled asthma significantly increases COVID-19-related mortality [8], and nonallergic asthma is associated with severe COVID-19 [9], whereas allergic asthma is not. The mechanism is not yet fully understood; however, the relationship between ACE2 receptor expression in the airway and Th2 inflammation degree with genetic predisposition may provide some clues [9]. Several studies postulated that inhaled corticosteroids can be safely used in patients with asthma in the COVID-19 era regardless of SARS-CoV-2 infection [10].
There is limited knowledge regarding the differences in COVID-19 severity among patients with allergic rhinitis (AR). However, it is necessary to note that COVID-19 symptoms may be mistaken for AR and their early recognition can be delayed in patients with AR because they share a few similar clinical symptoms. The European Academy of Allergy and Clinical Immunology provides a set of symptoms to distinguish COVID-19 from AR [11]. Smell dysfunction, taste dysfunction, dyspnea, and cough are the predominant symptoms of COVID-19, whereas AR predominantly manifests as sneezing, stuffy nose, and nasal pruritus with ocular itching or redness. Intranasal corticosteroids, the most effective single maintenance therapy for AR, can be safely used to manage AR, even during a SARS-CoV-2 infection [12].
The relationship between atopic dermatitis (AD) and COVID-19 outcomes remains controversial and inconclusive. Some studies in adults shown that AD is associated with an increased risk of COVID-19, whereas others reported that AD patients do not have a significantly increased risk of SARS-CoV-2 infection. The most part of managing AD involves maintaining skin hydration, removing exacerbating factors, and applying appropriate medications despite the COVID-19 pandemic. The continuous use of topical steroids as well as biologics such as dupilumab leads to better disease outcomes regardless of the presence of COVID-19 [13]. However, the use of systemic corticosteroids for 1 year reportedly increases one’s risk of COVID-19-related hospitalization [13].
SARS-CoV-2 is currently widespread and significantly less fatal than it was in 2020. However, the pandemic has remained unpredictable. We may still encounter new SARS-CoV-2 variants or other viruses that may create an even cumbersome situation. Lessons from the COVID-19 pandemic will help establish a rapid strategy for prioritizing management plans for patients with allergic diseases. In a pandemic, evidence-based measures for the management of allergic diseases should be established that consider the risk of viral infection and transmission, socioeconomic aspects, and healthcare accessibility.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
