Clinical characteristics of pediatric patients infected with SARS-CoV-2 versus common human coronaviruses: a national multicenter study
Article information
Abstract
Background
Human coronaviruses (HCoV) cause mild upper respiratory infections; however, in 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged, causing an acute respiratory disease pandemic. Coronaviruses exhibit marked epidemiological and clinical differences.
Purpose
This study compared the clinical, laboratory, and radiographic findings of children infected with SARS-CoV-2 versus HCoV.
Methods
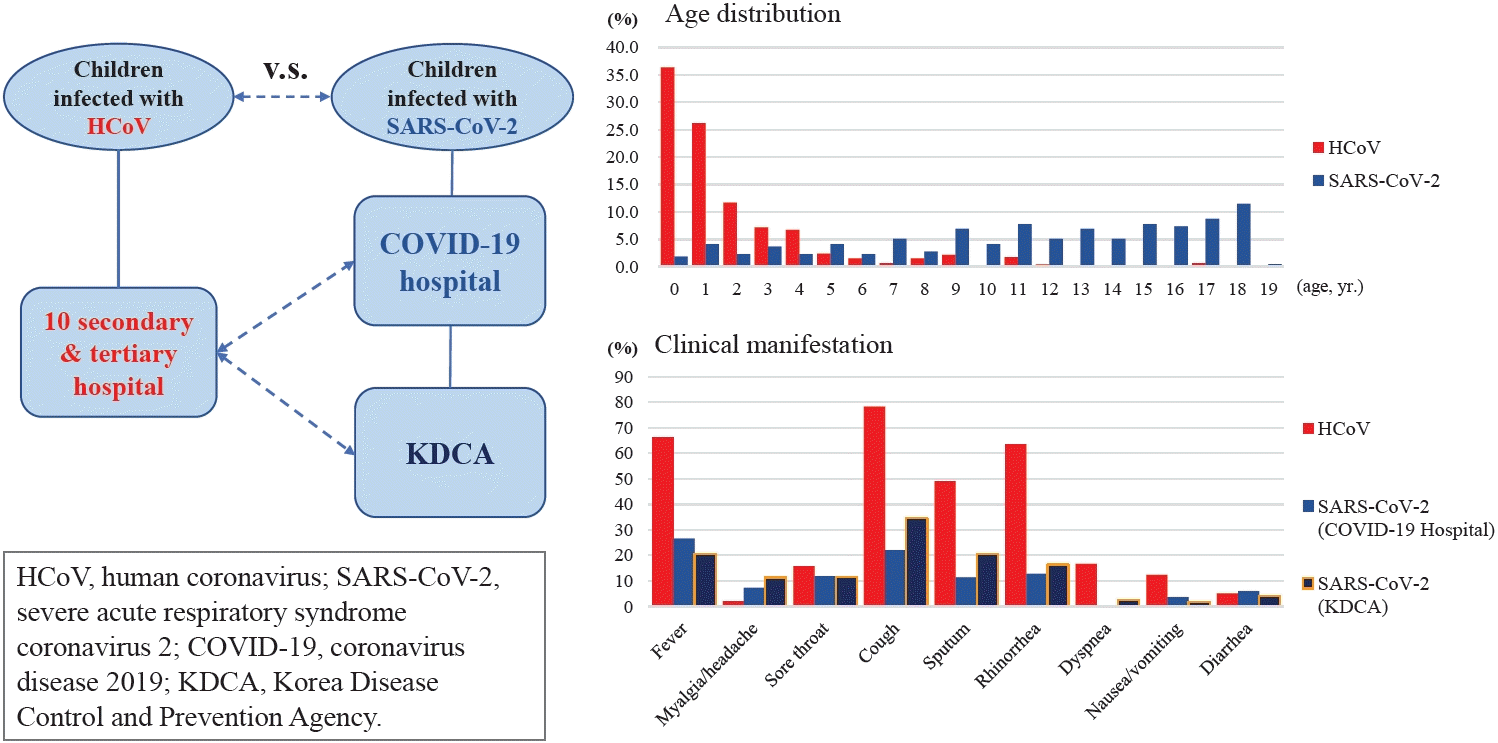
SARS-CoV-2 data were obtained from the Korea Disease Control and Prevention Agency (KDCA) registry and 4 dedicated coronavirus disease 2019 (COVID-19) hospitals. Medical records of children admitted with a single HCoV infection from January 2015 to March 2020 were collected from 10 secondary/tertiary hospitals. Clinical data included age, sex, underlying disease, symptoms, test results, imaging findings, treatment, and length of hospital stay.
Results
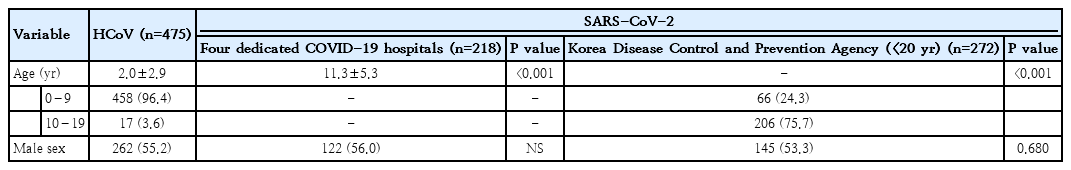
We compared the clinical characteristics of children infected with HCoV (n=475) to those of children infected with SARS-CoV-2 (272 from KDCA, 218 from COVID-19 hospitals). HCoV patients were younger than KDCA patients (older than 9 years:3.6% vs. 75.7%; P<0.001) and patients at COVID-19 hospitals (2.0±2.9 vs 11.3±5.3; P<0.001). Patients with SARS-CoV-2 infection had a lower rate of fever (26.6% vs. 66.7%; P<0.001) and fewer respiratory symptoms than those with HCoV infection. Clinical severity, as determined by oxygen therapy and medication usage, was worse in children with HCoV infection. Children and adolescents with SARS-CoV-2 had less severe symptoms.
Conclusion
Children and adolescents with COVID-19 had a milder clinical course and less severe disease than those with HCoV in terms of symptoms at admission, examination findings, and laboratory and radiology results.
Key message
Question: The clinical differences between severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and human coronaviruses (HCoV) in children remain unknown.
Finding: This study compared the clinical findings of children infected with SARS-CoV-2 versus HCoV. Its findings suggest that children and adolescents with SARS-CoV-2 have a milder clinical course than those with HCoV.
Meaning: The clinical course of children and adolescents with SARS-CoV-2 should be closely monitored during the coronavirus disease 2019 pandemic.
Graphical abstract
Introduction
Coronaviruses are members of the subfamily Coronavirinae in the family Coronaviridae and the order Nidovirales. The 4 commonly known human coronaviruses (HCoV) are HCoV-229E, HCoV-HKU1, HCoV-NL63, and HCoV-OC43 [1]. These HCoVs circulate globally, causing respiratory and intestinal infections in animals and humans; but human infection is usually mild and is mainly found in children. However, outbreaks of highly contagious and often fatal cases of severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) coronaviruses occurred in the early 2000s and the 2010s, respectively. In 2019, a novel, highly transmissible, pathogenic coronavirus emerged and caused a pandemic of acute respiratory disease named coronavirus disease 2019 (COVID-19) [2]; it was first reported in Wuhan, China, in December 2019 [3]. Similar to SARS or MERS, COVID-19 exhibits differences in the frequency of infection, severity, and clinical manifestations experienced by adults and children. The COVID-19 prevalence for children is lower than that for adults [4]. By June 1, 2021, 6,220 infections had occurred in individuals 0 to 9 years of age in Korea, and 10,064 infections had occurred in individuals 10 to 19 years of age in Korea, accounting for 4.42% and 7.15% of total cases, respectively. No deaths have been reported in these 2 age groups [5].
One study that examined adult patients 65 years or older, in China, with severe infection and comorbidities reported a high risk of death attributable to acute respiratory distress syndrome [6]. According to a case report and expert opinion [7] of 28 pediatric patients with COVID-19 in Wuhan, most of the infected children were asymptomatic or had a fever, dry cough, and fatigue; some reported upper respiratory and gastrointestinal symptoms. Most of them had a mild clinical course and recovered after 1 to 2 weeks; however, a few reported progressions to lower respiratory tract infection. Similarly, to date, most pediatric patients, with confirmed COVID-19, in Korea have had mild symptoms, and no deaths have been reported [8]. Recently, a case of severe COVID-19 pneumonia that rapidly progressed to respiratory distress in a child was reported in Korea [9].
HCoVs are one of the causes of pediatric acute respiratory infections, such as bronchiolitis, pneumonia, and croup [10]. HCoVs have been detected in 3.4% of children 1 month to 5 years of age with acute wheezing [11], and in 1.7% of children who were hospitalized for respiratory tract infections [12]. One prospective study revealed that HCoV infection was detected in 5.7% of children hospitalized for acute respiratory illness and in 7.1% of asymptomatic controls [13].
There are differences in the clinical manifestations and disease severity experienced by children and adults with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This pattern differs from that of most other respiratory viral infections, which have a higher prevalence and are usually more severe in children [14]. Therefore, we hypothesized that HCoV infection would be more severe than SARS-CoV-2 in children in terms of symptoms, laboratory findings, and chest imaging results. This study aimed to compare the clinical characteristics of children hospitalized with SARS-CoV-2 and those of children hospitalized with HCoV infection.
Methods
1. Study design and ethical approval statement
This is a retrospective cohort study to investigate the clinical characteristics of children and adolescent infected with HCoV and SARS-CoV-2. This study was approved by the Institutional Review Board of Dongguk University Ilsan Hospital (protocol No. 2020-12-024-001). The requirement for informed consent was waived due to the retrospective nature of the study
2. Patients infected with SARS-CoV-2
We obtained clinical information from SARS-CoV-2 patients using data provided by the Korea Disease Control and Prevention Agency (KDCA) about patients hospitalized for SARS-CoV-2 infection between February 1 and April 30, 2020. During the ongoing COVID-19 pandemic, testing and management of individuals with SARS-CoV-2 were performed in accordance with the guidelines of the Korean quarantine authorities [15]. Patients with suspected COVID-19 who tested positive for SARS-CoV-2 were transferred to a community treatment center or hospital to receive appropriate treatment according to their symptoms [16]. Therefore, all hospitalized COVID-19 patient data are managed by the KDCA and are publicly available. These data included sex and age distributed in 5-year units, along with limited blood test results. Clinical symptoms at admission included fever, cough, sputum, sore throat, rhinorrhea, myalgia, fatigue, dyspnea, headache, altered consciousness, nausea/vomiting, and diarrhea. The most common comorbidities were hypertension, diabetes, heart failure, chronic heart disease, asthma, chronic obstructive pulmonary disease, chronic renal disease, cancer, chronic hepatic disease, rheumatic/autoimmune disease, and dementia. Laboratory parameters included hemoglobin, hematocrit, and platelet and lymphocyte numbers. Additional disclosed information included whether the patient was admitted to the intensive care unit (ICU), used oxygen, or used a ventilator and/or extracorporeal membrane oxygenation. Children were analyzed separately (i.e., those younger than 20 years of age).
A database of children infected with SARS-CoV-2 admitted to 1 of 4 dedicated COVID-19 hospitals between February 1, 2020, and February 28, 2021, was also accessed to obtain additional information such as fever duration, auscultation findings, laboratory results (such as complete blood count and aspartate aminotransferase [AST], alanine transferase [ALT], lactate dehydrogenase [LDH], C-reactive protein [CRP], and procalcitonin [PCT] levels), and chest x-ray and chest computed tomography imaging results.
3. Patients infected with common HCoV
Patients infected with a single HCoV were included among patients hospitalized for respiratory infections at 10 secondary or tertiary hospitals between January 2015 and March 2020. All patients were tested for 9 respiratory pathogens and respiratory bacterial pathogens, including Mycoplasma pneumoniae. Patients with coinfection (bacteria or viruses other than human coronavirus) were excluded. The medical records of children who were admitted with a single HCoV infection were collected. Clinical data included age, sex, underlying disease, symptoms, laboratory test results, chest imaging findings, treatment, and length of hospital stay. This database included fever duration, symptoms such as cough, sputum, rhinorrhea, sore throat, fatigue, headache, and myalgia, as well as physical examination results. Laboratory results included complete blood count and AST, ALT, LDH, CRP, and PCT levels.
4. Statistical analysis
Three groups of patients infected with SARS-COV-2, sorted by data source and age, were analyzed, and compared with those infected with HCoV. Normally distributed data were analyzed using Student t test. Nonnormally distributed data were analyzed using the Mann–Whitney U test. All statistical analyses were performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). The results were deemed significant when P<0.05.
Results
1. Patient characteristics
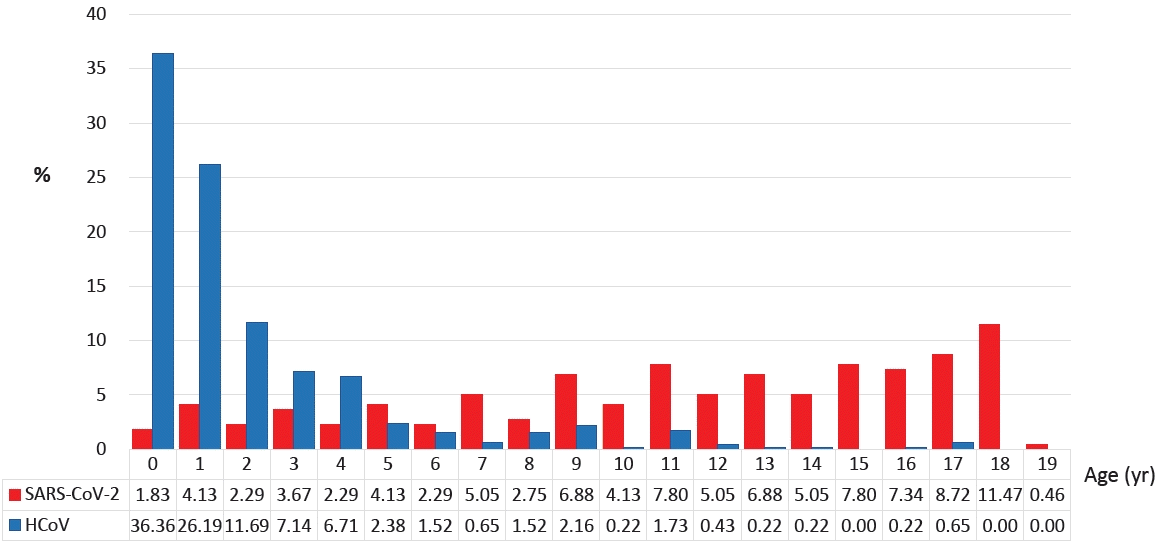
Of the 5,628 patients from the KDCA database, 272 patients younger than 20 years old and 218 patients from the 4 COVID-19 dedicated hospitals were included in our study. The 2 groups of patients infected with SARS-CoV-2 were compared to patients infected with HCoV (n=475). The basic characteristics of each group are listed in Table 1. Children infected with HCoV were younger than those infected with SARS-CoV-2, and the mean age of HCoV patients was younger than that of SARSCoV-2 patients from the 4 hospitals (2.0±2.9 years vs. 11.3±5.3 years, P<0.001) (Fig. 1). The proportion of children 0 to 9 years of age was higher in the HCoV group than in the SARS-CoV-2 group from the KDCA (96.4% vs. 24.3%, P<0.001). The proportion of males was higher in the SARS-CoV-2 group than in the HCoV group. The length of hospital stay was shorter for HCoV patients than for SARS-CoV-2 patients (4.0 days [range, 3.0–6.0 days] vs. 13.0 days [range, 10.0–19.0 days], P<0.001). The most common final diagnosis was respiratory disease in both groups, with pneumonia (19.6%) and bronchitis (18.7%) for HCoV, and bronchitis (18.1%) and croup (16.2%) for COVID 19 patients. Infective endocarditis, cerebellar ataxia, transverse myelitis, Idiopathic throbocytopenic purpura, erythema multiforme and Henoch schoniein purpura have been diagnosed only in patients infected with SARS-CoV-2 (data not shown).
2. Underlying disease
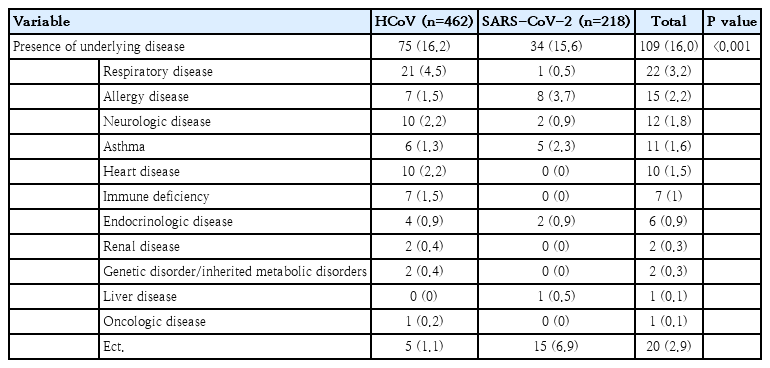
The proportion of underlying chronic disease among SARS-CoV-2 patients younger than 20 of age in the KDCA database was lower than that among HCoV patients (2.6% vs. 16.5%, P< 0.001). Chronic obstructive pulmonary diseases, such as bronchopulmonary dysplasia, bronchiectasis, and bronchiolitis obliterans, were the most common underlying chronic conditions of patients hospitalized with HCoV. However, data from the 4 hospitals showed that the rate of underlying disease for SARS-CoV-2 patients was 16.2%, which was similar to that of patients with HCoV. Allergic diseases, such as atopic dermatitis and allergic rhinitis, were the most common (3.7%), followed by asthma (2.3%) (Tables 2, 3).

Underlying diseases of patients infected with HCoV versus patients infected with SARS-CoV-2 from Central Disaster and Safety Countermeasure Headquarters
3. Clinical symptoms at admission
Although 96.8% of HCoV patients had symptoms at the time of hospitalization, only 52% and 61% of children and adolescents infected with SARS-CoV-2 (from KDCA and the 4 hospitals), respectively, reported symptoms. Fever on admission was more prevalent for HCoV patients (66.3%) than for SARS-CoV-2 patients from the 4 hospitals (26.6%) and the KDCA (20.6%) (P<0.001). The proportions of myalgia and headache were lower for HCoV patients (2.11%) than for SARS-CoV-2 patients; 4 hospitals, 7.3%; P=0.011 and the KDCA, 11.4%; P<0.001. Respiratory symptoms, including cough, sputum, and rhinorrhea, were more commonly observed in patients with HCoV than in all patients with COVID-19. About 16.7% of HCoV patients reported dyspnea; however, for SARS-CoV-2 patients, no patients from 4 hospitals and 2.6% from the KDCA database reported dyspnea (Table 4).

Clinical manifestation and physical examination findings of patients infected with HCoV versus SARS-CoV-2 at admission
Physical examination of HCoV patients indicated crackles (17.1%), wheezing (20.4%), and chest retraction (10.0%). However, wheezing did not occur in SARS-CoV-2 patient from the 4 hospitals (Table 4).
4. Laboratory and imaging findings
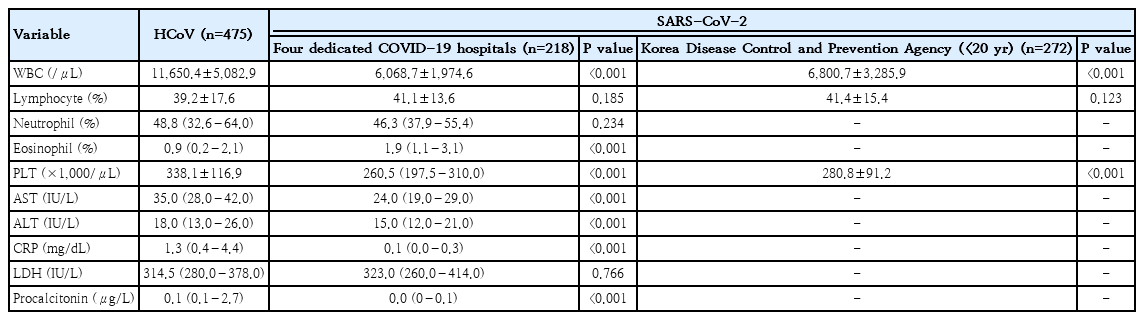
The laboratory findings are presented in Table 5. HCoV patients had higher white blood cell counts and platelet levels than SARS-CoV-2 patients. Lymphocyte differential counts did not differ between children infected with SARS-CoV-2 and children infected with HCoV. The following levels were higher in patients with HCoV than in children infected with SARS-CoV-2 from the 4 dedicated hospitals: AST (35.0 IU/L vs. 24.0 IU/L; standard deviation [SD], 7.0 vs. 5.0 IU/L; P<0.001); ALT (18.0 IU/L vs. 15.0 IU/L; SD, 6.5 vs. 4.5 IU/L; P<0.001); CRP (1.25 mg/L vs. 0.1 mg/L; SD, 2.0 mg/dL vs. 0.14 mg/dL; P<0.001); and PCT (0.11 µg/L vs. 0.03 µg/L; SD, 1.3 µg/L vs. 0.016 µg/L; P<0.001).
Although 63.1% of HCoV patients had normal radiographic findings, their proportion of abnormal radiographic findings, including bronchial infiltration, lobar consolidation, interstitial pneumonia, was higher than that of SARS-CoV-2 patients from the 4 dedicated hospitals (175 of 475 [36.8%] vs. 62 of 218 [28.4%], P=0.031). Among 218 patients infected with SARS-CoV-2 from the 4 dedicated hospitals, 68 patients underwent chest computed tomography; of these 68 patients, 72% had normal results, and 3% had ground-glass opacity (data not shown).
5. Disease severity
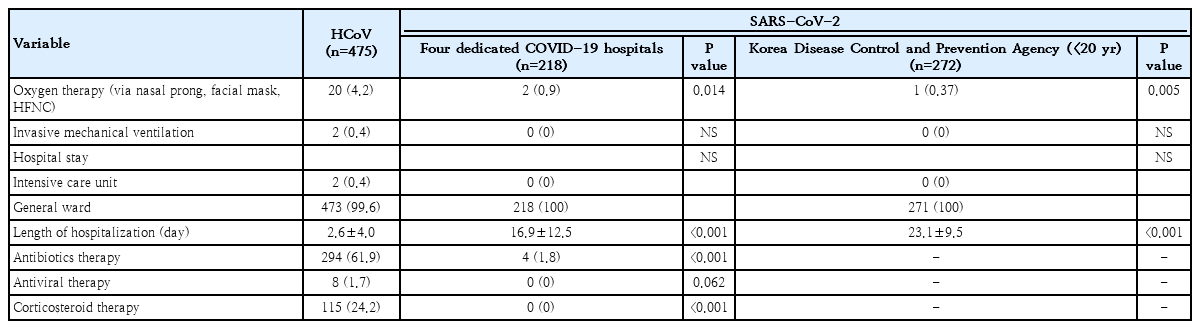
More HCoV patients required oxygen therapy during hospitalization than SARS-CoV-2 patients younger than 20 years of age from the 4 dedicated hospitals (4.2% vs. 0.9%, P<0.014) and the KDCA (4.2% vs. 0.37%, P<0.005). Although 0.42% of HCoV patients and 0.34% of SARS-CoV-2 patients of all ages in the KDCA database needed a mechanical ventilator, none of the SARS-CoV-2 patients younger than 20 years from the 4 dedicated hospitals and the KDCA database used a mechanical ventilator. A total of 0.4% of HCoV patients were admitted to the ICU; however, no SARS-CoV-2 patients younger than 20 years were admitted to the ICU. Antibiotic treatment rates were higher for HCoV patients than for SARS-CoV-2 patients younger than 20 years of age from the 4 hospitals and the KDCA (61.9% vs. 1.8%, P<0.001). Corticosteroids were used for 24.2% of HCoV patients, and antiviral agents were used for 1.7% of HCoV; however, neither agent was used for SARS-CoV-2 patients (Table 6).
Discussion
Although the pandemic novel coronavirus continues (at the time of writing this article), there is limited information about the clinical characteristics and course of infection in children. SARS-CoV-2 belongs to the same genetic lineage as HCoV, which is common in children [17]. Coronaviruses (there are 7 that are known to infect humans) can cause a spectrum of clinical presentations ranging from asymptomatic infection to severe illness and death [18].
Children had a lower incidence of COVID-19, a milder disease course, and a better prognosis than adults [19,20]. During our study, SARS-CoV-2 patients of all ages had several severe disease features; however, children and adolescents infected with SARS-CoV-2 or HCoV had mild symptoms.
According to studies of pediatric COVID-19 symptoms, the best-established characteristics have been the presence or absence of fever and cough; however, these symptoms have been recorded in only 30% of pediatric case reports [4]. These reports indicate that fever, cough, sore throat, rhinorrhea, and gastrointestinal symptoms are frequent symptoms [6]. Headaches and muscle aches, which are commonly observed in adults, have not been observed in children [4]. Pneumonia and symptoms requiring hospitalization accounted for approximately one-third of pediatric cases, whereas severe disease accounted for approximately 9% of pediatric cases [4]. Overall, most children with COVID-19 were asymptomatic or had mild symptoms, with some experiencing progression to severe infection [21,22]. During our study, SARS-CoV-2 patients also had a lower incidence of fever than HCoV patients; however, SARS-CoV-2 patients had higher incidences of headache and myalgia. A larger proportion of adults with SARS-CoV-2 exhibited these symptoms compared to children with SARS-CoV-2 (data not shown).
The characteristic laboratory results for adults with SARS-CoV-2 are not common in children. Lymphopenia has been reported as the most common sign for adults and is considered a potential indicator of COVID-19 severity [23-25]. However, it is present in only 17.5% of children [26]. This could be attributable to an immature immune system or because COVID-19 symptoms are less severe for those younger than 20 years of age [26]. During our study, SARS-CoV-2 patients of all ages had lower lymphocyte counts than HCoV patients (data not shown). However, differential white blood cell counts of pediatric SARS-CoV-2 patients indicative of lymphopenia and other conditions were similar to those of HCoV patients. Liver enzyme levels of children are frequently normal; however, they are abnormal in critically ill children with severe COVID-19 [27]. SARS-CoV-2 patients in our study who did not experience progression to severe disease also had normal AST and ALT values.
During our study, most x-ray imaging findings of SARS-CoV-2 patients were normal, and chest computed tomography performed for some patients showed mostly normal findings. According to a systematic review that examined the radiologic characteristics of children with COVID-19, the imaging findings of children were milder than those of adults [28]. Another study found that bilateral ground-glass opacities were the most prevalent findings [29]. These results are consistent with those of the present study.
Our study had some limitations. Children and adolescents with HCoV or SARS-CoV-2 have different hospitalization criteria. HCoV patients were hospitalized for treatment according to symptom severity, whereas patients with confirmed SARS-CoV-2 were hospitalized mainly for isolation purposes, regardless of symptoms, especially when the patients were children. Children with SARS-CoV-2 are typically asymptomatic, but some are hospitalized along with their infected parents for childcare purposes. This may have led to the overall impression that HCoV symptoms are more severe than those of SARS-CoV-2. The patients also had different discharge criteria. us patients may be discharged if their symptoms improve; however, for SARS-CoV-2 patients, quarantine release is essential. A patient hospitalized for SARS-CoV-2 can be discharged only if 2 consecutive polymerase chain reaction tests at least 24 hours apart at least 10 days after onset are confirmed to yield negative results, even if the fever and clinical symptoms improve [30]. This affected the length of hospital stay of pediatric SARS-CoV-2 patients. Finally, during this study, among patients with a common HCoV infection, those with coinfection with other viruses or bacteria were excluded from the study no coinfection was confirmed for SARS-CoV-2 patients. Therefore, it cannot be ruled out that SARS-CoV-2 patients may have had symptoms caused by other viral or bacterial infections. Studies have shown that 40% to 50% of children with SARS-CoV-2 were coinfected with another virus or bacteria [29,31].
Our study showed that children with SARS-CoV-2 had less severe symptoms than those with a common HCoV. However, because of the low prevalence of infection among children compared to adults, the clinical course of children with SARS-CoV-2 should be closely monitored during the coronavirus pandemic.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by a research grant for the project of the Korean Academy of Pediatric Allergy and Respiratory Disease.
Author contribution
Conceptualization: Kang EK, Kim CK, Yang HJ, Data curation: Sol IS, Lee E, Yang HJ, Yum HY, Lee MH, Chu MA, Kim HB, Seo JH, Shim JY, Ahn JY, Jang YY, Chung HL, Jung EH, Kim CH, Kang EK, Kim CK. Formal analysis: Kim CS. Methodology: Yang HJ, Lee YJ, Kim K, Kim BS, Lee KS, Han MY, Hong SJ, Kim JT; Software: Moon HJ, Sol IS. Investigation: Sol IS, Kang EK, Yang HJ. Writing - original draft: Sol IS, Kang EK. Writing - review & editing: Sol IS, Kang EK, Kim CK