Neurodevelopmental outcomes of preterm infants
Article information
Abstract
During the last several decades, the number of preterm infants has increased, and their survival rate has improved owing to advances in perinatal care. As more preterm infants survive, many studies examine their neurodevelopmental outcomes. This study aimed to summarize the neurodevelopmental outcomes of preterm infants according to gestational age at birth using a recently published meta-analysis. The prevalence of neurodevelopmental impairment and behavioral disorders decreased as gestational age and birth weight increased. Recent studies reported that proactive neonatal treatment of periviable preterm infants (gestational age, 22–24 weeks) could improve their prognosis. Moderate and late preterm infants reportedly have less severe disease than very preterm infants; nonetheless, they still experience adverse neurodevelopmental outcomes compared to term infants. Neonatal morbidities such as bronchopulmonary dysplasia and retinopathy of prematurity are associated with poor neurodevelopmental outcomes. Despite improvements in neonatal care, prematurity is still associated with poor neurodevelopmental outcomes. To ensure timely intervention, the establishment of a follow-up system for premature infants is necessary.
Key message
· Among survivors, 60.9% of infants born at 22 weeks’ gestation had moderate to severe impairments, whereas 50.3% born at 23 weeks’ and 42.2% at 24 weeks’ gestation had moderate to severe impairments.
· Moderate and late preterm infants reportedly have less severe disease than very preterm infants, but they still experience adverse neurodevelopmental outcomes.
· The careful follow-up and early detection of developmental problems in these patients are required.
Introduction
Preterm infant is defined as an infant born prior to 37 weeks’ gestation. Such infants are categorized based on gestational age (GA) as extremely preterm (EP; <28 weeks), very preterm (VP; 28–31 weeks), moderate preterm (32–33 weeks), and late preterm (34–36 weeks). During the past few decades, the number of preterm infants has increased, while the survival rate has improved due to advances in care in the neonatal intensive care unit (NICU) worldwide [1-3]. Recently, improved outcomes of infants born during the periviable period (22–23 weeks’ GA) have also been reported in some countries [4]. As more preterm infants survive, increasing studies of neurodevelopmental outcomes are conducted. Most studies focused on EP/VP, and outcomes were worse than those of full-term infants [5-7]. However, debate persists about the outcomes of moderate- to late-preterm (MLPT) infants [8-10]. This study aimed to summarize the neurodevelopmental outcomes of preterm infants according to their GA at birth using recently published meta-analyses. It also reviews recent research into the neurodevelopment of Korean preterm infants.
Methods
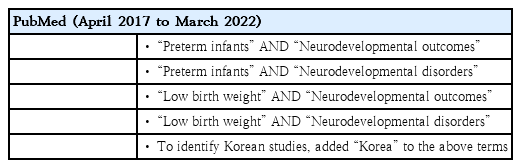
A literature search was performed of PubMed using the keywords listed in Table 1. The results were narrowed down to meta-analyses published between April 2017 and March 2022. For a review of Korean studies, “Korea” was added to the keywords and the same period applied. Articles that were not related to the neurodevelopmental outcomes of preterm infants or low birth weight (LBW) infants were excluded. Several widely used assessment tools are reviewed shortly before the systematic reviews are summarized.
Results
1. Neurodevelopment assessment
The Bayley Scales of Infant and Toddler Development, Third Edition (BSID III) is among the most common tools used to assess the neurodevelopment of preterm infants. It assesses 5 domains of development in children aged 1–42 months: cognition, language (receptive and expressive communication), motor (gross and fine), social-emotional, and adaptive behavior. The first 3 domains were assessed by a trained examiner, whereas information about the last two was obtained from the child’s main caregiver [11]. The fourth edition was recently updated. In the new edition, the number of items in each domain is decreased, and the examination takes approximately 30% less time than the third edition [12].
The Gross Motor Function Classification System (GMFCS) is widely used to assess motor development and functional level in infants and children. This system focuses on self-initiated movements, especially sitting, transfer, and mobility. It consists of 5 levels, and the definition of each was expanded and revised according to patient age. Children with higher GMFCS levels have more movement limitations and may have a more unfavorable prognosis [13].
The Wechsler Intelligence Scale for Children is an intelligence test for children aged 6–16 years. The most recent version is the fifth edition published in 2014 [14], which was developed to enable a comprehensive assessment of a child’s general intellectual functioning in various situations, for example, identifying students with learning disabilities, intellectual disability, or giftedness; assessing cognitive processing strengths and weaknesses; and evaluating the impact of brain injuries [14].
The Korean Developmental Screening Test for Infants and Children (K-DST) is used as a developmental screening tool in the National Health Screening Program for Infants and Children. The tool is a parent-reported test composed of 6 domains: gross motor, fine motor, cognition, language, social skills, and self-help. It can be used for infants and children aged 4–71 months [15].
Several studies have reported the proportion of children diagnosed with autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) using national health insurance data classified according to the standard International Classification of Diseases [9,16].
2. Meta-analyses of neurodevelopmental outcomes of preterm infants or LBW infants
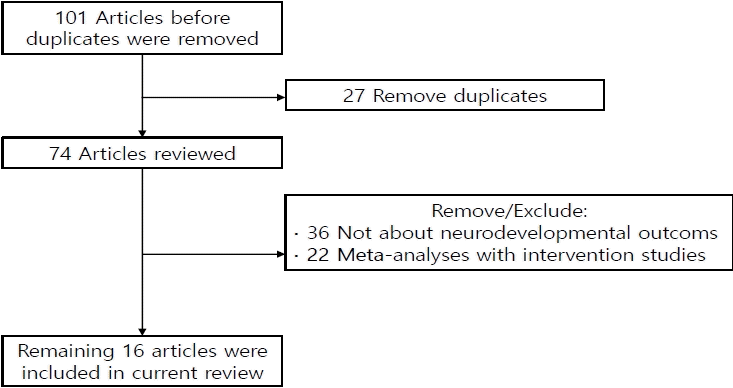
One hundred one studies were identified in the initial search process; of them, 27 duplicated studies were removed, while another 36 studies were manually excluded for irrelevancy. Among the 38 remaining studies, 22 focused on interventional studies improving neurodevelopmental outcomes and 16 focused on the natural outcomes of preterm infants or LBW infants (Fig. 1, Table 2).
1) Neurodevelopment of preterm infants born at periviable GA
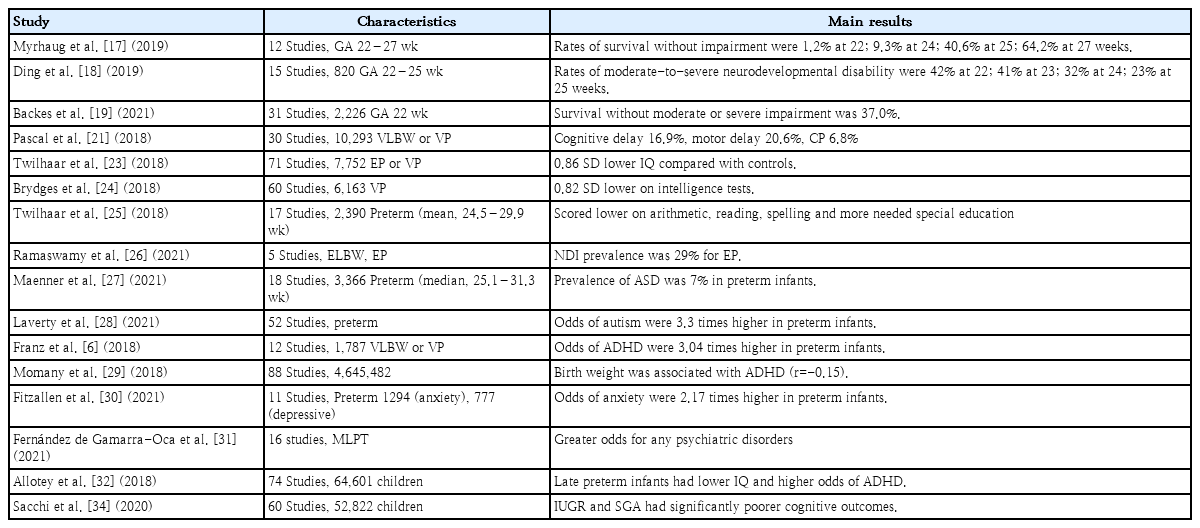
Several meta-analyses examined the neurodevelopmental outcomes of infants born in the periviable period (22 and 23 weeks’ GA). In a meta-analysis of 65 studies, the mean survival rate of infants born at 22 weeks’ GA was nearly 0% of all births, 7.3% of live births, and 24.1% of infants admitted to the NICU. Among survivors, 60.9% of infants born at 22 weeks’ GA had moderate to severe impairments, whereas 50.3% of those born at 23 weeks’ GA and 42.2% born at 24 weeks’ GA had moderate to severe impairment (Fig. 2) [17]. Another study that assessed survivors aged 4–10 years showed that moderate to severe NDI rates were 42.0% at 22 weeks’ GA, 41.0% at 23 weeks’ GA, 32.0% at 24 weeks’ GA, and 23.0% at 25 weeks’ GA [18]. The prognosis was better for infants who were provided proactive neonatal treatment, even when they were born at 22 weeks’ GA. The pooled prevalence of survival was 29.0%; in 5 studies that assessed survival with moderate or severe NDI, the overall rate was 63.0% [19]. As studies have reported that proactive neonatal treatment for periviable preterm infants could improve prognosis, more research is required to establish evidence-based treatment protocols [4]. In addition, the recently published neonatal resuscitation guidelines did not specify GA or birth weights for withholding or withdrawing resuscitation, unlike the previous guidelines [20].
2) Neurodevelopment of VP or very LBW infants
The prevalence of NDI during the first 2 years of life decreased as GA or birth weight increased. In a meta-analysis of VP or very LBW (VLBW) infants born after 2006, the pooled prevalence of a moderate to severe cognitive delay was 10.9% for 26–27 weeks’ GA and 5.8% for 28–32 weeks’ GA. Moderate to severe motor delay also showed a similar trend: 12.0% for 26–27 weeks’ GA and 6.3% for 28%–32% weeks’ GA. The prevalence of cerebral palsy assessed using GMFCS in VLBW infants was 6.8%, but the prevalence was higher in extremely LBW (<1,000 g, ELBW) infants (8.4%) than in those with birth weights of 1,000–1,500 g (4.2%) [21]. The prevalence of cerebral palsy in VLBW infants has not notably improved over the last 3 decades [22]. Two meta-analyses found that children or adolescents born with EP or VP also had large deficits in intelligence, and no improvement was observed during the last several decades [23,24]. VP and VLBW infants also have considerable academic difficulties and special educational needs [25]. Studies reported that bronchopulmonary dysplasia (BPD) is an important factor in cognitive outcomes and is suggested a lowering of its incidence [23,25]. Another study that evaluated the outcomes of EP or ELBW in developing countries found that the rate of NDI was 17% (7%–34%) for ELBW and 29% (23%–37%) for EP infants, although the certainty of evidence was very low to low [26].
3) Behavioral disorders and mental health of VP infants
VP or VLBW infants are at an increased risk of neurodevelopmental disorders such as ASD or ADHD. A meta-analysis of 3,366 preterm infants (mean GA, 28.0 weeks) estimated that the overall prevalence of ASD was 7% (95% confidence interval [CI], 4%–9%) [7]. This was higher than that of the general population, in which 1 of every 44 children (2.3%) aged 8 years has been diagnosed with ASD [27]. A previous study reported that preterm infants were estimated to have an odds of ASD that was 3.3 times higher than general population [28]. VP and VLBW infants are also at higher risk of developing ADHD than term infants [6,29]. The odds ratio (OR) was 3.04 (95% CI, 1.56–3.26) for VP/VLBW and EP/ELBW infants, while EP/ELBW infants had a higher odds (4.05; 95% CI, 2.38–6.87) than VP/VLBW infants (2.25; 95% CI, 1.56–3.26) [6]. Multiple factors can affect causal pathways of neurodevelopmental disorders in premature infants. A nonoptimal intrauterine environment, fetal immune response, exogenous stress during the perinatal period, and genetic factors can affect brain development and interfere with neuronal organization [6,7]. Another meta-analysis reported that preterm-born children also had a higher prevalence of anxiety and specific phobia, but not depressive disorders, than term-born children [30].
4) Neurodevelopmental outcomes of MLPT infants
MLPT infants are reportedly less severely affected than VP infants but still experience adverse neurodevelopmental outcomes. In a meta-analysis of MLPT infants, no differences were observed in cognitive function between adults born at term and MLPT term. However, they had higher odds for any psychiatric (OR, 1.14; 95% CI, 1.04–1.24), substance use (OR, 1.16; 95% CI, 1.06–1.27), and psychotic disorders (OR, 1.40; 95% CI, 1.07–1.83) [31]. Another study reported that late preterm infants had a lower intelligence quotient (IQ) and higher odds of ADHD (OR, 1.3; 95% CI, 1.1–1.5) [32]. As the number of MLPT infants has increased over the last few decades, more studies are needed, and primary care physicians should cautiously observe their neurodevelopmental outcomes [33].
5) Cognitive outcomes of intrauterine growth restriction
A study on cognitive outcomes of intrauterine growth restriction and small for GA revealed a significantly increased risk of poorer cognitive outcomes in both preterm and term-born children compared with children who were appropriate for GA [34]. The biological mechanisms are not clearly explained, but the authors speculated that suboptimal trophic inputs due to placental insufficiency or undernutrition may affect in utero brain development [34].
3. Korean studies
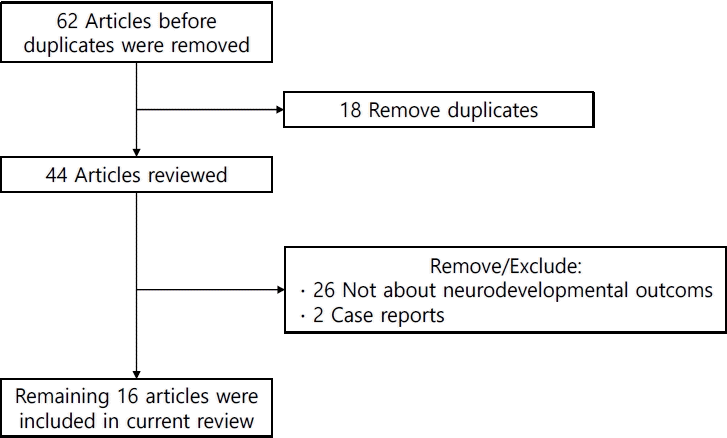
Sixty-two studies were identified in the initial search process. Of them, 18 duplicate studies were removed, while 28 were excluded manually for irrelevancy (Fig. 3). Among the 16 remaining studies (Table 3), 3 used Korean National Health Insurance Service (KNHIS) data and 4 used Korean Neonatal Network (KNN) data. The KNN is a nationwide registry of VLBW infants. The registry was established in 2013; more than 18,000 infants were registered. Healthcare workers report infant demographics and neonatal outcomes (e.g., survival, BPD, intraventricular hemorrhage), and long-term outcomes (growth and developmental outcomes at 18 and 36 months of corrected age [CA]) based on medical record review. The registry uses the K-DST and BSID to monitor developmental outcomes [35].
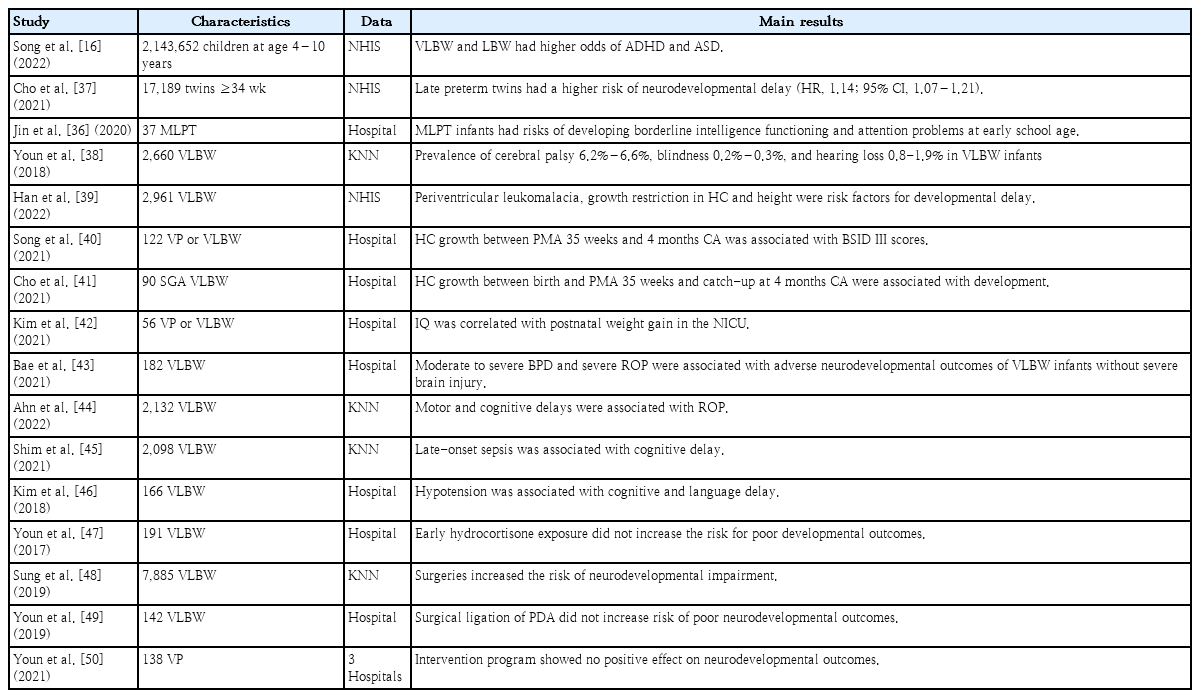
Two studies reported the neurodevelopmental outcomes of late preterm infants or LBW infants (1.5–2.4 kg) using KNHIS data [17,36]. One study revealed that VLBW and LBW are associated with ADHD or ASD. A stronger association between ADHD/ASD and birth weight was observed as birth weight decreased. In a subgroup analysis, LBW infants without a perinatal history showed similar results [17]. Another study of late preterm twins found that they were at increased risk for any neurological or neurodevelopmental delay (hazard ratio, 1.14; 95% CI, 1.07–1.21) compared to term twins. In this study, the hazard ratio of ASD and ADHD was significantly increased (1.17; 95% CI, 1.01–1.36) in preterm twins [37]. A study of MLPT infants born in a single institution showed similar results of a risk of developing borderline intelligence functioning and attention problems [36]. A study of KNN data reported the overall prevalence of cerebral palsy (6.2%–6.6%), blindness (0.2%–0.3%), and hearing loss (0.8%–1.9%) in VLBW infants born in 2013–2014 [38].
Several studies assessed the associations between perinatal factors and neurodevelopmental outcomes. A study using KNHIS data reported that periventricular leukomalacia and growth restrictions in head circumference and height were independent risk factors for developmental delay [39]. Others focused on the association between growth and development. Song et al. [40] reported that head circumference growth between a postmenstrual age (PMA) of 35 weeks and 4 months of CA was associated with BSID III scores appropriate for GA preterm infants. Cho et al. [41] revealed that z score changes in head circumference between birth and a PMA of 35 weeks and achieving head growth catchup by 4 months of CA were significantly associated with favorable neurodevelopmental outcomes (BSID III at 18 months) in small GA preterm infants. Another study showed that weight gain in the NICU was positively correlated with full-scale IQ at school age [42].
Two studies reported that retinopathy of prematurity (ROP) was associated with unfavorable outcomes of BSID III at 18 months’ CA [43,44]. The authors explain this result using several hypotheses. First, visual impairment can negatively affect synaptic reorganization following brain damage. Second, previous studies reported that severe ROP is associated with delayed white matter maturation and reduced brain volume [43]. Third, as ROP is caused by poor development of the blood vessels in the retina, which is part of the central nervous system (CNS), it may be associated with abnormal CNS function [44]. Shim et al. [45] reported that lateonset sepsis was associated with cognitive delay at 18–24 months’ CA (OR, 1.48; 95% CI, 1.02–2.16), while Kim et al. [46] reported that hypotension in the first week of life increased the OR (3.53; 95% CI, 1.34–9.32) and poor neurodevelopmental outcomes (BSID III) in VLBW infants,.
In contrast, exposure to hydrocortisone during the first week in VLBW infants was not associated with adverse developmental outcomes at 18 months’ CA (BSID III) [47]. Two studies examined the association between surgical intervention and development. Sung et al. [48] reported that the risk of NDI (BSID III) increased as the number of surgeries increased. It was impossible to specify the reasons underlying morbidities or anesthetic medications. In contrast, in a single-center study, the surgical ligation of patent ductus arteriosus was not associated with poor neurodevelopmental outcomes (BSID III) at 18months’ CA [49]. An interventional study aimed to improve developmental outcomes. In a multicenter randomized control trial, the authors tried to examine whether a preventive intervention program of 4 home visits by a nurse could improve developmental outcomes, but no significant differences were noted between the control and intervention groups [50].
Discussion and conclusion
In this review of premature infants, the prognosis for survival without NDI was noticeably poorer at a younger GA. MLPT infants also had developmental outcome concerns. Survival free of major impairments among EP infants has increased during the last several decades but remains high among children born at <25 weeks’ GA [51].
Several interventions have been attempted to improve neurodevelopmental outcomes. Prophylactic early recombinant human erythropoietin (rhEPO) is expected to provide neuroprotection for preterm infants. Previous studies reported that rhEPO improves the cognitive development of VP but not EP infants. Moreover, there was no significant effect on any secondary outcome, such as psychomotor development, cerebral palsy, or severe visual or hearing impairment [52-55]. The effects of prebiotic and probiotic supplementation and micro- (zinc, iodine) or macronutrient (amino acid) supplementation on the neurodevelopment of preterm infants have been studied, but the evidence remains insufficient [56-60].
To identify NDI in time and provide timely interventions, a follow-up system for premature infants must be established. In Korea, the Ministry of Health and Welfare started a pilot program that funds coordinator nurses who promote the regular follow-up of VLBW or VP infants at 4, 8, 18, 24, and 36 months’ CA [61]. Furthermore, the government planned to set a new statistics system using KNN and other national big data (e.g., KNHIS), which would enable efficient monitoring until adulthood [62]. With the sharing of established information with primary care physicians, appropriate monitoring and early intervention can be achieved.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.