Update on eosinophilic gastrointestinal disease beyond eosinophilic esophagitis in children
Article information
Abstract
Eosinophilic gastrointestinal disease (EGID) is an emerging condition worldwide in both children and adults, although it is considered rare. EGID has been underestimated owing to its underdiagnosis in the past, and its prevalence has recently increased. The diagnosis of EGID is based on histopathologic findings of endoscopic mucosal biopsy in which the total number of infiltrating eosinophils in each segment of the gastrointestinal tract is determined in patients with chronic or recurrent gastrointestinal symptoms despite a lack of consensus or guidelines on the definition of tissue eosinophilia except for eosinophilic esophagitis. Laboratory findings, including peripheral eosinophilia and increased fecal calprotectin levels, may be additional clues for detection of EGID in practice. Individualized treatment strategies, including adequate dietary modification and pharmacological therapy, may improve clinical outcomes of pediatric patients with EGID.
Key message
· Eosinophilic gastrointestinal disease (EGID) is uncommon, with a prevalence of 1–30/100,000 in the general population; however, it is increasing worldwide.
· The diagnosis of EGID is based on histopathological findings of endoscopic mucosal biopsy in which tissue eosinophils are counted in each gastrointestinal tract segment of patients with chronic or recurrent gastrointestinal symptoms.
· Individualized treatment strategies, including adequate dietary and pharmacological therapy, may help improve outcomes of children with EGID.
Graphical Abstract.
Introduction
Eosinophilic gastrointestinal disease (EGID) was first described in 1937 by Kaijser [1]. EGID is a rare heterogeneous disease group of mixed type of gastrointestinal (GI) allergic disease that selectively affect one or more segments of the GI tract with eosinophilic inflammation in the absence of other causes [2].
Although eosinophilic esophagitis (EoE) is a well-defined disease entity with recommended guidelines, EGID distal to the esophagus, such as eosinophilic gastritis (EoG), eosinophilic enteritis (EoN), and eosinophilic colitis (EoC), remains unclear, with limited evidence and no consensus guidelines for adults or children [3].
Therefore, this article reviews EGID with focus on EGID beyond EoE with updated evidence, particularly in pediatric patients.
Epidemiology
Since international consensus is lacking on the diagnostic criteria of EGID except EoE, its exact prevalence is unknown and difficult to estimate, although the prevalence of EoE is reportedly 34.4–52 per 100,000 in the general population of Western countries [4,5]. Nevertheless, the prevalence of EGID was estimated at 1–30 per 100,000 people in the general population [6]. Furthermore, a study published in 2016 reported the estimated prevalence of EoG, EoG/EoN, and EoC as being 6.3/100,000, 8.4/100,000, and 3.3/100,000, respectively, in the United States [7].
Except for the serosal type of EGID, there is a sex-based difference in the prevalence of EGID with a male to female ratio of 3:2 [8]. In addition, EGID is more prevalent in adults than ].n children and more common in Caucasians than in other races [8)
However, in a recent study, the prevalence of EGID was reportedly 19.4/100,000 in children, similar to the 17.9/100,000 in adults [7]. In this study, while the prevalence of EoG was higher in adult patients, there was no age difference in EoC; moreover, the prevalence of EoG/EoN was highest in children younger than 5 years of age [7].
Although the prevalence of EoE in Asia is approximately 20/100,000, data are lacking on the prevalence of EGID including EoG, EoG/EoN, or EoC in Asian countries [9]. However, it is assumed to be less common than in Western countries [9].
Since EGID has been underdiagnosed to date, data on its prevalence have been underestimated. However, it seems to be rising due to better recognition and increasing interest [6,10]. In a nationwide multicenter study of Korean children published in 2020, EoE was diagnosed in 1.5% (14 of 910 pediatric patients) based on upper endoscopy and mucosal biopsies, whereas EoG/EoN involving the esophagus was detected in 1.3% (12 of 910 children) [10]. Both EoE and EGID distal to the esophagus are uncommon but emerging disease conditions of interest worldwide [9].
Clinical features of EGID in children
EGID causes GI symptoms such as nausea, vomiting, abdominal pain, and diarrhea in pediatric patients [6]. Therefore, it may be one of important organic causes of consideration when a child complains of these nonspecific GI symptoms.
In children, EGID usually presents with various GI symptoms depending on the involved GI segment and the extent of eosinophilic inflammation. EGID is generally classified into mucosal, muscular, and serosal types according to the most involved layer of the intestinal wall because the anatomic distribution of eosinophilic inflammation is related to its clinical features [8]. The mucosal type of EGID is the most common, manifesting with nonspecific GI symptoms such as nausea, vomiting, diarrhea, weight loss, anemia, and protein-losing enteropathy. The muscular type of EGID, the second most common, often leads to intestinal obstruction or intussusception. The serosal type is uncommon and develops abruptly in a single episode accompanied by eosinophilic ascites. Unlike the serosal type, the disease course of the mucosal and muscular types of EGID is chronic and relapsing. As only mucosal biopsies are taken during endoscopy and overlap can occur between 2 or 3 types of EGID, it is often difficult to classify the exact type [8].
Pathogenesis of EGID
The etiology and pathogenesis of EGID have not yet been confirmed. EGID is a mixed type of food allergy [11]. It may involve both immunoglobulin E (IgE)- and non-IgE-mediated hypersensitivity to food allergens because of hypereosinophilia in the peripheral blood and GI tissue, increased serum IgE levels, the presence of elevated inflammatory mediators produced by eosinophils such as eotaxin, eosinophilic cationic protein (ECP), interleukin (IL)-3, IL-5, and granulocyte macrophage colonystimulating factor (GM-CSF) in GI tissue, delayed Th2 type allergic reactions, and clinical response to steroid therapy [11,12].
Eosinophils, which normally comprise approximately 1%–4% of leukocytes in the blood, are multifunctional cells that play an essential role in innate and adaptive immunity during the initiation, progression, and resolution of immune responses. Eosinophilic inflammatory activity and selective eosinophil recruitment from the bloodstream into the tissue of the GI tract in EGID are complex processes regulated by inflammatory mediators, including IL-3, IL-4, IL-5, IL-13, and GM-CSF [13,14]. In particular, IL-5 regulates eosinophilic recruitment and infiltration into the GI tract in association with eotaxin [15]. Eosinophils release 4 cytotoxic granule proteins, including ECP, major basic protein (MBP), eosinophil-derived neurotoxin, and eosinophil peroxidase on degranulation. MBP and other inflammatory mediators directly activate mast cells, which may act as the eosinophil-mast cell axis, causing tissue inflammation and damage as part of the pathogenesis of EGID [16]. IgE may be involved in mast cell degranulation in EGID through IgE-induced mast cell–mediated mechanisms of eosinophilic chemotaxis [13,17].
Therefore, eosinophil-mediated immune responses in the pathogenesis of EGID is a complex process related to the production of food antigen-specific IgE antibodies, Th2 cell activation, and eosinophil-activating inflammatory cytokine overexpression with an excessive tissue eosinophil load in the tissues of the GI tract [13].
Laboratory findings of EGID in children
Increased peripheral eosinophil counts and serum total IgE levels were suggested as laboratory clues for detecting EGID in about half of affected patients [11]. While peripheral eosinophilia is not a sensitive marker for tissue eosinophilia in patients with functional GI disorders, an increased peripheral eosinophil count may be a beneficial finding in those with EGID [11]. However, the significance of this finding is limited because peripheral eosinophil counts or serum IgE levels can be within the normal range in other patients [14].
In our study published in 2020, increased fecal calprotectin (FCal) levels indicated a higher possibility of EGID [18] FCal is an intracellular cytoplasmic protein of neutrophils with bacteriostatic properties [19]. The FCal level is proportional to neutrophil migration into the GI tract and positively correlates with leukocyte excretion in the feces [20]. Because exposure to specific food allergens leads to GI inflammation in patients with EGID, and an increased FCal level was shown in EGID [18]. Thus, FCal may be a useful noninvasive marker for intestinal inflammation that can differentiate between organic GI diseases, including EGID, and inflammatory bowel disease [18]. Further evaluations including endoscopy with mucosal biopsy can be considered in patients with increased FCal levels.
Diagnosis of EGID in children
In practice, it is difficult to detect and diagnose EGID since the diagnosis requires histological confirmation of tissue eosinophilia on invasive endoscopic biopsy (Fig. 1) [21]. Furthermore, endoscopic findings of EGID except EoE can be normal or nonspecific, although non-Helicobacter pylori and nongastrotoxic drug ulcers may be detected during endoscopy in some pediatric patients with EGID [22].
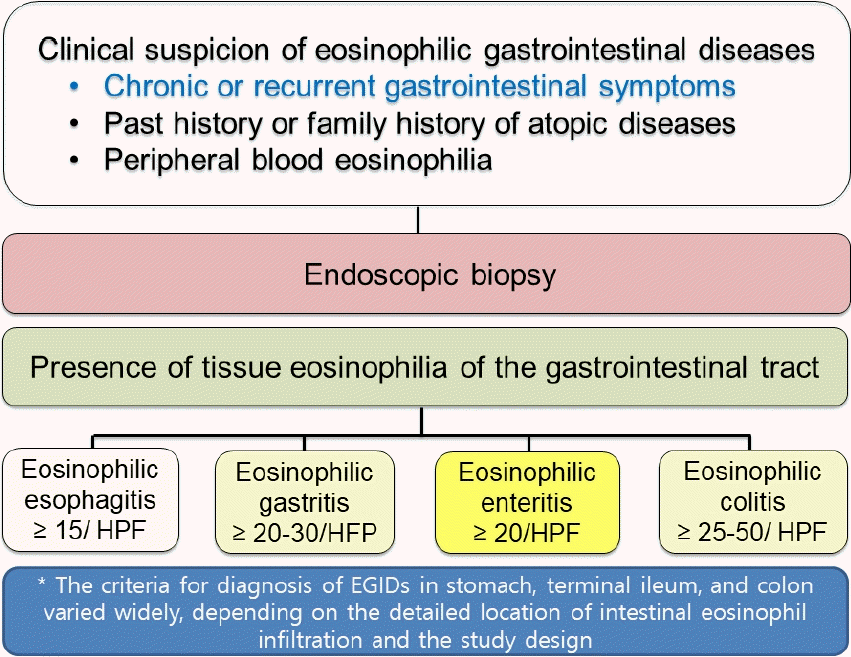
Diagnostic approach to eosinophilic gastrointestinal diseases in children. HPF, high-power field; EGID, eosinophilic gastrointestinal disease.
In 1990, Talley et al. [23] suggested the following 3 criteria for the diagnosis of EGID: (1) presence of chronic or recurrent GI symptoms; (2) histopathological findings of significant eosinophilic infiltration into the mucosa or muscle layer of the GI tract in at least one biopsy sample or the presence of a high eosinophil count in ascites fluid in serosal type cases; and (3) the exclusion of other causes of tissue eosinophilia of the GI tract for the differential diagnosis (Fig. 1) [4,8,23,24]. Nevertheless, welldefined criteria for the diagnosis of EGID (except EoE) are still lacking.
For the histopathologic diagnosis of EGID, it is essential to confirm tissue eosinophilia of the GI system [23]. However, a universal consensus is lacking on the exact number of tissue eosinophils in each GI segment to fulfill the diagnostic criteria of EGID except for EoE. Previous studies suggested that the total number of infiltrating eosinophils per high-power field (HPF) in the GI mucosa should be ≥15/ HPF for EoE, ≥20–30/HPF for EoG, ≥20/HPF for EoN, and ≥25–30/HPF for EoE in at least one biopsy specimen in suspected cases [24]. Nevertheless, debate persists about the exact numbers of infiltration eosinophils in each segment of the GI tract for confirming the diagnosis of EGID beyond EoE. For example, the U.S. Food and Drug Administration has accepted ≥30 eosinophils/HPF in ≥5 HPF in the stomach and in ≥3 HPF in the duodenum as cutoff values for diagnosing EoG/EoN in some clinical trials, while other studies suggested lower or higher cutoffs [25]. The criteria for the diagnosis of EGID in the terminal ileum and colon also varied widely (20–50 eosinophils/HPF) depending on the detailed location of intestinal eosinophil infiltration and the study design [6,21,26,27]. A recent pediatric study of EGID in children applied ≥50/HPF for the right-sided colon, ≥35/HPF for the transverse colon, and ≥25/HPF for the left-sided colon [27].
Furthermore, it should not be overlooked that eosinophils can normally be present to some extent in the intestinal mucosa of GI biopsy specimens in children and adults and in any acute or chronic inflammatory disease conditions as a component of mixed inflammation [28,29].
Due to the lack of published international guidelines or established diagnostic criteria for EGID, its diagnosis should be made carefully. However, the diagnosis would be relatively easy to make in cases of clinical symptoms with prominently infiltrating tissue eosinophils in the GI mucosa on biopsy [25]. Moreover, histological findings of eosinophils infiltrating the epithelium, the presence of eosinophil aggregates or microabscesses, eosinophils showing extensive degranulation, and eosinophils within the crypt or muscularis mucosae can be additional clues supporting the diagnosis of EGID [29].
Treatment of EGID in children
There are no universally accepted treatment strategies for EGID because international consensus and clinical guidelines are lacking except for EoE. In practice, the treatment of EGID is usually individualized for each patient and determined according to symptoms and response time, using a step-up or step-down process in pediatric EGID patients [8].
Despite the lack of confirmed treatment options, recommended timing for initiating treatment, and treatment duration owing to limited data, dietary and/or pharmacologic therapies have been applied in practice to manage EGID in pediatric patients (Table 1).
1. Dietary therapy
Because EGID is a food allergy, dietary modification strategies such as a targeted elimination diet, the empirical 6-food elimination diet, and an elemental diet have been used to treat it [13,29,30]. The targeted elimination diet is based on positive results from food allergy tests such as a specific IgE and skin prick test, whereas an empirical elimination diet known as the 6-food elimination diet targets the restriction of 6 foods including milk, soy, egg, wheat, nuts, and seafood [8]. In clinical practice, the targeted elimination diet may be beneficial when a small number of food allergens test positive, while empirical 6-food elimination diets may be helpful when no allergens are detected or too many are positive on a food allergy test [13]. The elemental diet may be indicated in severe cases that are refractory to targeted or empirical elimination diets [8,2].
However, to date, randomized controlled studies are lacking on dietary therapy of EGID, and most are small or case series [13,30]. Therefore, well-designed studies of the dietary management of EGID are lacking. Accordingly, the efficacy of dietary therapy in managing EGID beyond EoE is controversial [13]. Nevertheless, there is some evidence that dietary modifications may effectively treat EGID. Clinical and histological remission was shown with both the 6-food elimination diet and the elemental diet for 6 weeks in an adult study [31]. In a pediatric study, about 82% of 30 children with EoG/EoN showed a clinical response without histological confirmation when the empirical elimination diet or elemental diet was applied [2].
The clinical approach of dietary therapy and modification to manage EGID in pediatric patients should be carefully decided on a case-by-case basis considering the clinical features and EGID type in the individual, positive results of food allergy tests, and compliance and adherence to different elimination diets.
2. Pharmacologic therapy
Drug therapy strategies for EGID in pediatric patients are controversial owing to insufficient data.
Proton pump inhibitors (PPIs) can be used to manage EGID, even in children. In a pediatric study, PPI therapy with lansoprazole 10 mg/day improved tissue eosinophilia of the duodenum in children with EoG/EoN [32]. The mechanism of the effect of PPI in EGID has been suggested that acid suppression improves clinical symptoms and mucosal inflammation regardless of the presence of gastroesophageal reflux disease and leads to a reduction of tissue eosinophils and inhibition of IL-4 and IL-13 activity [13,32].
Montelukast is a selective leukotriene-4 receptor antagonist that was previously used to treat allergic rhinitis and asthma [33]. It also effectively induces and maintains the remission of EoG/EoN in both adult and pediatric patients at a daily dose of 4–10 mg/day, although results vary among different age groups [34]. Clinical and histological responses were noted within 2–4 weeks after the start of montelukast therapy, and remission was maintained for more than 12 months in most studies, but this remains controversial [34-36]. Therefore, further studies are needed to ensure the long-term efficacy and safety of montelukast therapy for EoG/EoN. Nevertheless, montelukast monotherapy and/or its combination with low-dose systemic steroids may be beneficial in practice for managing EoG/EoN in both adults and children [36].
Ketotifen is a first-generation antihistaminic drug that has clinical and/or histological effects in some case reports or case series in conjunction with montelukast or steroids, but additional data are required to confirm its long-term efficacy [36-38]. Ketotifen monotherapy is not recommended in EGID due to a lack of evidence [13,39].
Oral sodium cromolyn, a mast cell stabilizer that blocks the release of mast cell mediators, can be used for weeks to more than 12 months, but its efficacy is controversial, as clinical and histological remission was observed in some cases but not in others [36].
Systemic steroids are key pharmacological therapies that can induce but not maintain remission in EGID. Systemic steroids may act in EGID by suppressing cytokines such as IL-3, IL-4, IL-5, and GM-CSF, induce eosinophil apoptosis, and reduce peripheral eosinophilia and tissue eosinophil infiltration [13,36,40]. Although debate persists on the proper treatment dosage and duration, oral prednisone 0.5–2 mg/kg/day (initial dose of 30–40 mg/day; max, 60 mg/day) for 2 weeks tapered over 6–8 weeks may be used in practice [13,36]. Systemic steroids are the most effective treatment, with clinical remission occurring within 2–3 weeks after the initiation of therapy in more than 80%–90% of patients with EGID in most studies, while the other 20% remained steroid-dependent [13,36,41]. In Korean children, while systemic steroid treatment resulted in a clinical response without relapse in 11 of 19 patients with EGID, relapse was noted in 21.1% after the cessation of steroid therapy, and no response to steroid therapy was also noted in another 21.1% [42]. Despite the strong therapeutic effect of systemic steroid therapy, it should be cautiously prescribed because of the lack of randomized controlled studies and clinical guidelines to support its use in practice in addition to potential serious adverse drug effects [13]. Moreover, a low dose of prednisone to maintain remission is not recommended due to a variety of adverse effects despite the possibility of disease relapse [13,36].
Other potential experimental drugs introduced in a few case reports may include immunosuppressant, such as azathioprine or 6-mercaptopurine in steroid-dependent refractory EoG/EoN cases, anti-tumor necrosis factor monoclonal antibody (infliximab or adalimumab), anti-IgE antibody (omalizumab), antiIL-5 antibody (mepolizumab), and Th2 inhibitors (suplatast tosilate) [36,43,44].
Individualized appropriate therapeutic strategies, including dietary modifications such as the targeted elimination diet, empirical elimination diet, and elemental diet, as well as medications such as montelukast, ketotifen, or systemic steroid therapy, may be beneficial for managing pediatric patients with EGID despite insufficient data [6,13,41].
Natural history of EGID in children
To date, limited data are available on the natural course of EGID. According to a recent report by de Chambrun et al. [45] in 2018, 43 adults with EoG/EoN were followed up from 1988 to 2009. In this study, patients with EoG/EoN showed a benign disease course, but recurrent and chronic diseases were observed in more than 50% of them; spontaneous remission without relapse in 40%, relapse in 37%, and chronic disease in 21% [45]. Peripheral eosinophilia at the initial diagnosis was related to an increased risk of relapse [45]. In a Korean study in children with EGID, relapse was observed in 21.1%, and the presence of gastroduodenal ulcer as well as steroid resistance were related to relapse in pediatric patients with EGID [42].
Unfortunately, no studies have examined the long-term prognosis of EGID except EoE. The majority of EoE cases in children may persist throughout adulthood; in fact, relapse was present in 32.5%–49.2% of patients based on the data from several studies [5]. Moreover, a higher risk of esophageal stricture was associated with delayed diagnosis [5,46]. The interval of a delay in diagnosis after symptom onset was 1.2–3.5 years in children versus 3.0–8.0 years in adults [5], and the risk of esophageal stricture increased about 9% annually until EoE was diagnosed [46]. Another small group study of the long-term natural history of childhood-onset EoE reported that esophageal stricture developed in 83% of 6 pediatric EoE patients after a mean 7.8 years of follow-up [47].
To ensure better clinical outcomes, it may be important to diagnose and treat EGID properly as early as possible, especially in pediatric patients since their symptoms may not improve without treatment and may lead to malabsorption, malnutrition, and growth failure [5].
Conclusion
Although EGID has been underestimated, its prevalence is recently increasing worldwide, even in children. Although consensus is lacking on the definition of tissue eosinophilia except for EoE, the diagnosis of EGID is based on histopathologic findings of the affected GI tract segment by counting of the number of infiltrating eosinophils in patients with GI symptoms. As a delayed diagnosis and treatment of EGID may lead to poor outcomes in children, the early detection of its occurrence and relapse may be important. Noninvasive markers such as peripheral eosinophilia and increased FCal concentration may be helpful in the early detection of EGID in children. Because overall data from well-designed high-quality studies are still limited and standardized clinical guidelines for pediatric EGID are lacking except for EoE, individualized treatment including dietary and drug therapy may be beneficial for pediatric EGID patients. Furthermore, large-scale well-designed studies as well as clinical guidelines with international consensus may be required in the future to increase our understanding of EGID in children.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.


