Pathogenetic and etiologic considerations of febrile seizures
Article information
Abstract
Febrile seizure (FS), which occurs in febrile children without underlying health problems, is the most common type of seizure disorder in children. The suggested pathogenesis of FS derived from several animal and human studies is multifactorial and debatable. Neuronal hyperexcitability, which develops during inflammatory responses that accompany fever, provokes seizures. However, the exact role of each inflammatory mediator (e.g., cytokines) is undefined in terms of the connection between systemic or local inflammation and the central nervous system, and the mechanisms by which cytokines increase neuronal excitability remain unclear. In contrast, the cause of fever in most children with FS is usually mild respiratory virus infection (e.g., rhinovirus, influenza virus, adenovirus, and enterovirus) rather than severe bacterial infections. In temperate regions, the major causative respiratory viruses seem to mirror seasonally prevalent respiratory viruses in the community. Therefore, vigorous efforts to identify the causative pathogen of fever may not be necessary in children with FS. Genetic factors seem to play a role in neuronal hyperexcitability, and some types of genetic variation have been identified in several genes encoding ion channels of neurons that participate in neuronal excitation. Although most children with FS have benign outcomes, some characteristics such as complex FS, febrile status epilepticus, consecutive afebrile seizures, and the presence of neurodevelopmental disabilities may require further genetic and neurologic evaluations.
Key message
∙ Inflammatory responses accompanying fever increase neuronal excitability in the central nervous system, which in turn provokes seizures.
∙ Fever in children with febrile seizures is usually caused by common respiratory viruses, the distributions of which match those of seasonal community-acquired respiratory tract infections.
∙ Several genetic variations in ion channels seem associated with neuronal hyperexcitability in children with febrile seizures.
Introduction
Febrile seizure (FS) is defined by the American Academy of Pediatrics as a seizure accompanied by fever without a central nervous system (CNS) infection that occurs in children aged 6–60 months [1]. The estimated prevalence of FS in healthy children is 2%–5% in Western countries [2] versus 7%–8% in Korea and Japan [3,4]. FS is further divided into simple and complex FS [1]. Simple FS is defined as a generalized seizure lasting for <15 min that does not recur within 24 hours. Complex FS is defined as a seizure that is focal, is prolonged (≥15 minutes), or recurs within 24 hours. Simple FS comprises approximately 70%– 80% of cases [5-10], and children experiencing simple FS show no evidence of increased risk for mortality, neurodevelopmental complications, and subsequent epilepsy compared to the general population [1]. Therefore, further neurologic evaluations are not recommended for most children presenting with FS. Instead, the identification and treatment of the fever’s etiology should be prioritized in children presenting with FS during the acute febrile phase [1]. Although it is well known that viral infections rather than severe bacterial infections are the major causes of fever in children with FS [8,10], investigations of the viral etiology in children with FS have not been extensively performed. Moreover, considering that <10% of children experience FS, the seizure-provoking potential of various viruses may differ, and individual host factors might influence seizure occurrence among children infected with the same virus.
In this review, the causative viruses of fever in children with FS and individual host genetic factors prone to FS are addressed, starting with a discussion of the pathogenesis of seizures in febrile children.
Pathogenesis of febrile seizures
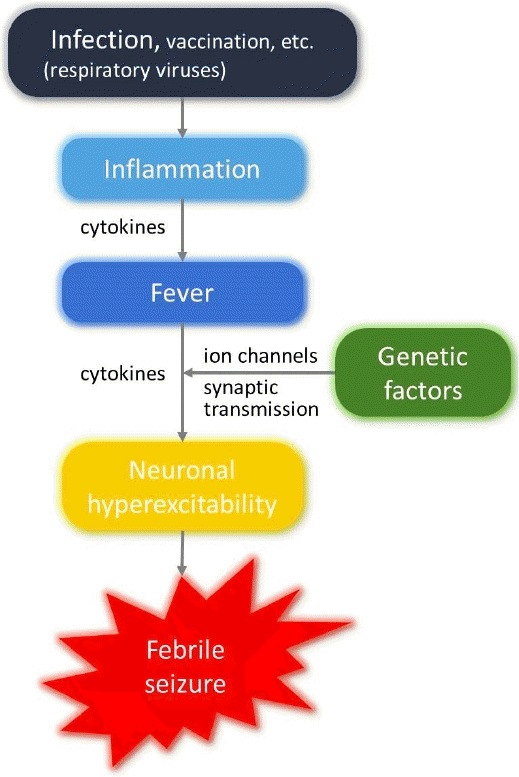
Among healthy children experiencing seizures accompanied by fever, only 6% are older than 60 months [11,12], as infants and young children with an immature CNS are more vulnerable to developing seizures than adolescents and adults with a mature CNS [13]. Notably, myelination occurs rapidly during the first 5 years of life, and a child’s total brain volume reaches approximately 90% of the average adult’s brain volume by 6 years of age [14]. Therefore, the prevalent age range of FS matches the rapid CNS development period. In the vulnerable immature CNS, increased neuronal excitability promotes seizures, and cytokines produced and released during acute inflammatory responses accompanying fever play a role in increasing neuronal excitability (Fig. 1) [15]. Inflammatory responses outside the CNS increase cytokine concentrations in the CNS (neuro-immune network), and the released cytokines trigger neuronal hyperexcitability in the CNS (cytokine roles in the brain parenchyma) to generate FS. This concept can be applied to the generation of afebrile seizures or epilepsy accompanied by various types of inflammation with non-infectious causes, including trauma, toxic injury, hypoxic injury, and autoimmune reactions [16]. Individual host genetic factors should also be considered, as only a small proportion of young children experience FS (Fig. 1). In addition, a family history of FS is consistently reported as a risk factor for FS [11]. Genetic epilepsy with febrile seizures plus (GEFS+) is representative of genetic epilepsy syndromes initially presenting with a phenotype of FS [17]. However, the pathogenesis of FS seems theoretical and hypothetical.
1. The neuro-immune network
Previously, the role of cytokines in the pathogenesis of FS was dubious [18]. Endogenous pyrogens among cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α), induce the synthesis of cyclooxygenase 2 (COX2), which in turn produces prostaglandin E2 (PGE2) in the brain perivascular cells and vascular endothelial cells [19]. Lipophilic PGE2 passes through the blood-brain barrier (BBB) and stimulates hypothalamic neurons [19]. Various brain cells, including neurons and glial cells, express receptors for PGE2, which regulates neuronal excitability in the brain parenchyma and increases body temperature [20]. Because cytokines are too large to penetrate the BBB, PGE2 produced by the stimulation of bloodborne cytokines was expected to increase neuronal excitability and contribute to FS development [18]. However, considering that nonsteroidal antiinflammatory drugs, which suppress PGE2 production by inhibiting the actions of COX2, could not prevent seizure occurrence [21], other factors beyond PGE2 provoke FS.
The cells constituting the BBB express pattern recognition receptors as well as receptors and transporters for several cytokines [22]. During immune stimulation, the modulation of cytokine transporters causes cytokines to traverse the BBB without structurally destroying it, and immune substances, including PGE2 and cytokines, can be produced and secreted directly into the interstitial space of the brain by BBB cells [22]. Because neurons and glial cells also possess cytokine receptors, they sequentially produce several types of cytokines [15,23]; as a result, both extrinsic and intrinsic cytokines can affect neuronal excitability. In addition to traversing the BBB, peripheral sensory nerves participate in the neuro-immune network system, of which the vagus nerve is representative. Vagal sensory neurons express receptors for immune substances, such as cytokines and prostaglandins [24]. Peripheral inflammation directly activates vagal afferent neurons and subsequently activates CNS neurons [24]. In the CNS, neurotransmitters activate glial and endothelial cells to secrete cytokines that induce inflammatory responses, and dysregulated inflammation may cause neuronal hyperexcitability and seizures [24]. Consequently, cytokines may increase neuronal excitability and provoke seizures in the absence of direct CNS invasion of pathogens, disruptive BBB changes, and definite brain parenchymal inflammation.
2. Roles of cytokines in the brain parenchyma
Cytokines bind to receptors on neurons and glial cells and regulate neuronal excitability via modifying ion channels and synaptic neurotransmission [15,23]. Standard proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, showed generally inhibitory effects on voltage-gated ion channels, resulting in neuroprotective effects rather than hyperexcitability [15]. For ligand-gated ion channels, some researchers reported that IL-1β provoked seizures by activating neuronal N-methyl-D-aspartate (NMDA) receptors and inhibiting neuronal γ-aminobutyric acid (GABA) receptors and astrocyte uptake of glutamate [23,25]. However, others reported enhanced effects of GABA receptors by IL-1β as well as conflicting synaptic effects of IL-1β on neuronal excitability [15]. IL-6 has inhibitory effects on NMDA receptors [15]. TNF-α tends to activate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors and inhibit GABA receptors [15,23]. TNF-α also provoked glutamate release and inhibited glutamate uptake by glial cells [25]. However, most of these data were obtained from experimental studies using rodents, whereas the results of in vivo and in vitro studies in humans were mixed. The effects of each cytokine on each ion channel vary according to subtype, number, and genetic variations of the ion channels on the target cells as well as target cell types, targeted brain regions, different cytokine concentrations, and exposure times to cytokines [15].
For children with FS, 2 meta-analyses of the association between cytokine concentrations and FS occurrence were reported [26,27]. One study reported that concentrations of IL-1β and IL-6 in the blood and cerebrospinal fluid showed no significant associations with FS occurrence [26]. In contrast, the other study reported significantly elevated concentrations of IL-1β and IL-6 in children with FS versus controls [27]. The main drawback of these meta-analyses was the small numbers of included studies and included children with FS: each included only 4–100 children with FS, and only 1–5 studies were meta-analyzed for each cytokine [26,27]. Moreover, most of the investigated cytokines showed conflicting results in terms of their association with seizure occurrence among studies [26]. After these meta-analyses, conflicting results on the role of IL-6 in FS development were further reported [28,29]. Higher concentrations of IL-4 and IL-10, which are anti-inflammatory or regulatory cytokines, were reported in children with FS versus febrile children without seizures [28,30]. This increase in anti-inflammatory cytokines seemed to represent a compensated state for inflammatory responses causing FS. In summary, cytokines exhibiting a consistently significant association with FS occurrence have not yet been identified.
Cytokines have a short half-life (usually <10 minutes) and maintain low systemic concentrations (usually <10 pg/mL) [31]; therefore, sampling timing and storage methods that prevent enzymatic degradation influence their measured concentrations. The presence of cytokine inhibitors, for example, soluble IL-1 receptor antagonist (IL-1RA) against IL-1β, interferes with the quantitative measurement of cytokine concentrations using immunoassay tests [31]. Accuracy and sensitivity vary among the various methods for measuring cytokine concentrations, and highly sensitive multiplexed methods have been developed [32]. Therefore, a well-designed study should be planned to identify cytokines that are significantly associated with FS occurrence while considering appropriate sample types, sampling times, sample storage and transportation methods, and testing methods. Considering that most episodes of FS occur within 24 hours after the initiation of fever [7], cytokines participating in innate immune responses, apart from adaptive immune responses, may be associated with FS occurrence.
Infectious pathogens associated with febrile seizures
Theoretically, if specific seizure-prone pathogens are identified in febrile children, FS may be prevented by the early identification and treatment of specific pathogenic infections.
1. Prior to polymerase chain reaction tests
Over the past several decades, viral infections were usually reported as the causes of fever in children with FS [8,10]. With regard to the clinical diagnosis of the cause of fever, upper respiratory tract infection (URI) was the most frequent, diagnosed in 60%–86% of children with FS [6,8,10,33]. Therefore, respiratory viruses should be the major cause of fever in children with FS. Among bacterial infections, urinary tract infection, occult bacteremia, and bacterial meningitis were diagnosed only in 1.1%–4.1%, 0.0%–2.9%, and 0.0%–1.4%, respectively, among tested children [6,8,10,33].
Viral cultures, antigen detection tests, and serological tests were conventionally used before the introduction of polymerase chain reaction (PCR) tests [6,8,34,35]. Using these test methods, the influenza virus was most frequently detected in children with FS, followed by adenovirus, parainfluenza virus, and enterovirus [6,34,35]. In studies performed in Singapore and Hong Kong, influenza virus infection was more significantly associated with FS than other respiratory virus infections [34,35]. In contrast, some researchers reported a lower detection rate of influenza virus than enterovirus, adenovirus, parainfluenza virus, and respiratory syncytial virus (RSV) in children with FS [8,10]. Considering that seasonal variations of dominantly circulating respiratory viruses in the community are not prominent in tropical versus temperate regions, the role of influenza virus during the winter may be offset by other prevalent viruses during the spring, summer, and autumn seasons in temperate regions. After the identification of human herpes virus-6 (HHV-6) as a cause of exanthem subitum in children, HHV-6 was considered the most common cause of FS in Western countries [36]. However, its role in FS occurrence was reportedly less prominent in Asian than Western countries [36]. Although a recent meta-analysis reported that HHV-6 was detected in 21% of children with FS [37], the detection rates of HHV-6 in children with FS were comparable to those in febrile children without seizures and in healthy children in several studies [36,38]. Moreover, rhinovirus, the most frequent pathogen in communityacquired URI, was usually excluded from investigation [6,10]. A number of existing serotypes and the lack of a common group antigen made antigen detection tests and serological tests for rhinovirus impossible [39]. New diagnostic modalities, such as PCR tests, exhibit increased sensitivity for detecting respiratory viruses compared to conventional test methods; they can identify respiratory viruses that are not detected by conventional test methods [39]. The introduction of PCR tests should reveal different results in terms of the distribution of respiratory viruses as causes of fever in children with FS.
2. Use of PCR tests
Detection rates for respiratory viruses in children with FS increased from 47.2%–63.0% using conventional test methods to 71.3%–82.7% using PCR tests [5,7-10,40]. Rhinovirus became a major causative virus in children with FS in addition to the previously known influenza virus, adenovirus, enterovirus, and parainfluenza virus (Table 1) [7,9,40-42]. In 2 studies, influenza virus, parainfluenza virus, and coronavirus infections were significantly more frequent in children with FS than in asymptomatic children or children with URI [9,41]. One study compared the distributions of respiratory viruses between children with FS and those with confirmed or suspected URI [41]. The febrile children without seizures, in whom multiplex PCR testing was omitted at the treating physician’s discretion, and the afebrile children who were included in the control group owing to their respiratory symptoms may have led to selection bias. In another study, children with FS were compared to afebrile healthy children [9]. The authors explained that the different potentials for fever development of each respiratory virus may cause different rates of FS occurrence [9]. Therefore, the independent contribution of each respiratory virus to the development of FS should be determined by comparing infected children with FS and similarly infected febrile children not presenting with seizures.
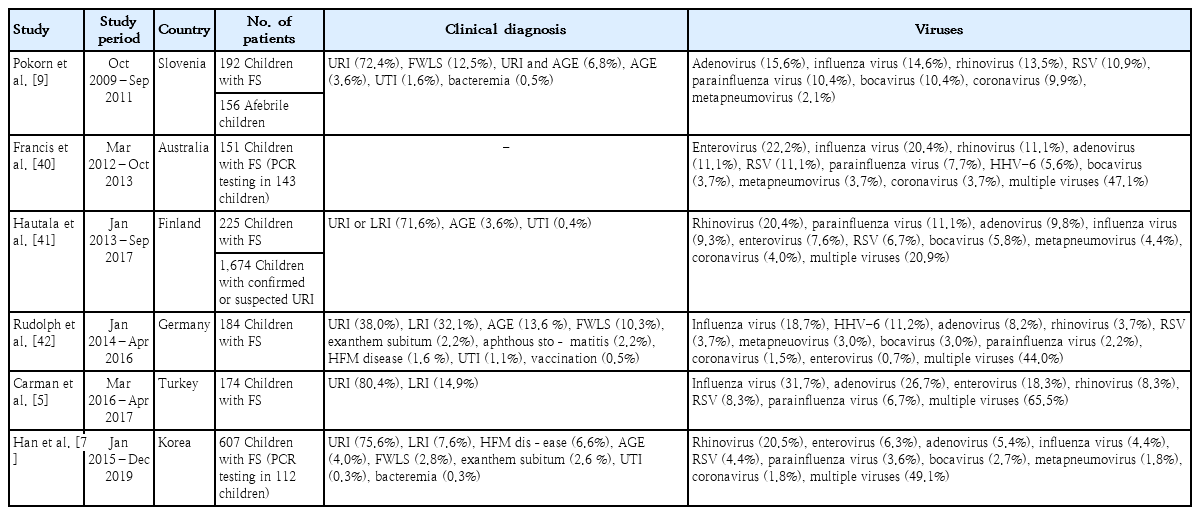
Recent studies reporting respiratory viruses detected by polymerase chain reaction (PCR) in children with febrile seizures
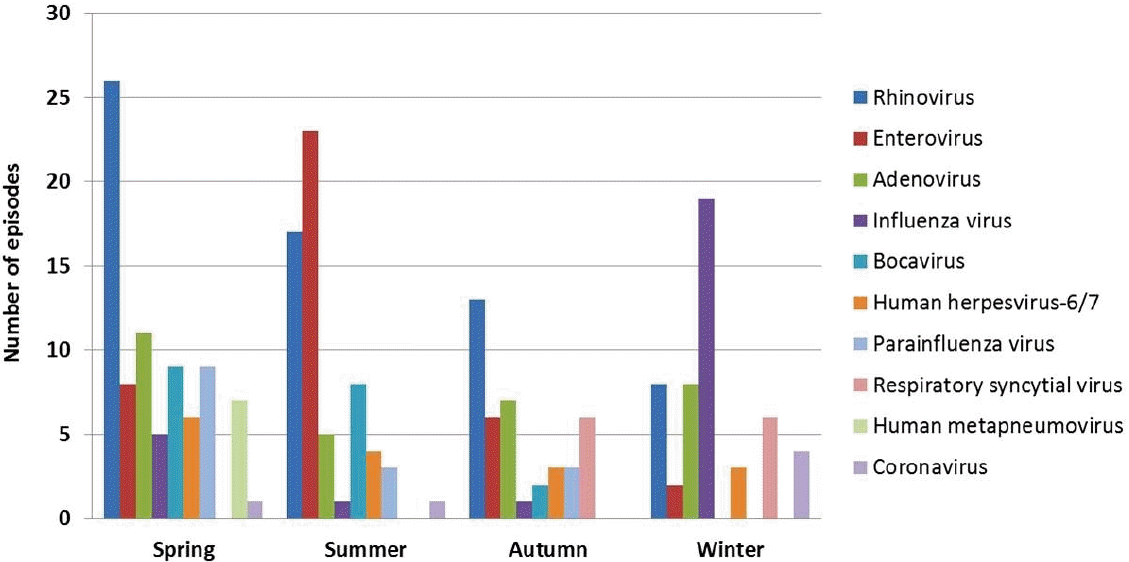
Although rhinovirus, adenovirus, influenza virus, and enterovirus were the most frequently detected causes of fever in several studies, their frequencies differed among studies (Table 1). As mentioned above, seasons could lead to discrepancies in the frequencies of respiratory viruses in temperate regions owing to seasonal variations in each virus. Different immune responses and cytokine profiles have been observed among respiratory viruses and subtypes, even among the same virus types [43]. These differences in immune responses may lead to different clinical severities and potentials for developing fever and FS according to viral types and subtypes. Therefore, the order of frequency of respiratory viruses detected in children with FS should vary by season when FS develops and the dominant circulating virus and its subtype in the community during the season. Our previous study showed that seasonal distributions of respiratory viruses detected in Korean children with FS were the same as those observed in community-acquired respiratory tract infections in Korea (Fig. 2) [7]. In Japan, which has 4 seasons like Korea, the incidence of FS was higher in winter and summer than in spring and autumn [44], representing the prevalence of various respiratory viruses, including influenza virus, in winter and the prevalence of enterovirus in summer.
Multiplex PCR tests have higher sensitivity than conventional viral tests; therefore, false positive results due to colonized or nonviable viruses and the codetection of multiple viruses should be addressed [45]. For children with FS, multiple respiratory viruses were simultaneously detected in 20.9%–65.5% [5,7,9,40-42]. However, it is currently impossible to determine which of the detected respiratory viruses is the true cause of the current fever and FS. Although the comparison of viral loads using a quantitative or semiquantitative PCR test may discriminate a true pathogen from codetected viruses, the colonization rates, thresholds of viral loads for symptom development, and associations between viral load and symptom severity vary among viruses [45,46]. Moreover, studies of the relationship between viral load and the development of FS are scarce, and a previous study reported comparable cycle threshold values of detected viruses through PCR tests between children with FS and controls [9]. When both children who tested positive for a single virus infection and those who tested positive for multiple viruses were examined, the most frequently detected viruses were identical [5,7,40]. A recent study reported that the characteristics and outcomes of FS were comparable between children who tested positive for a single virus infection and those who tested positive for multiple viruses [42]. Therefore, among viruses codetected via multiplex PCR tests, it is reasonable that the prevalent virus in the community at the time that the FS developed is considered the primary cause of the FS.
Differences in seizure characteristics and outcomes due to different respiratory viruses have rarely been reported in children with FS. Only marginal differences in the proportion of complex FS, seizure duration, and seizure recurrence among viruses were reported [7,9,40]. For influenza virus infection, infected children tended to be older than those infected by other respiratory viruses, and 22.7% of influenza-infected children presenting with seizures accompanied by fever were aged ≥5 years [5,12]. However, patient age was not significantly associated with seizure outcomes in these patients [12].
In summary, the significant potential for seizures and the grave seizure outcomes of specific respiratory viruses remains unclear, although rhinovirus, influenza virus, adenovirus, enterovirus, and parainfluenza virus were detected most frequently in children with FS. In temperate regions, efforts to identify the causative virus of fever in children with FS should not be cost-effective, especially if we consider the generally benign nature of FS, the similar prevalence of respiratory viruses to that of circulating viruses in the community, and the lack of specific antiviral treatment except for influenza virus.
Testing for influenza is necessary among children presenting with FS during influenza seasons, especially if they have risk factors for severe influenza. Different immune response profiles according to infected virus types and subtypes may explain the aforementioned inconsistent results regarding the roles of cytokines in FS. Further studies to establish the pathogenesis of FS should be designed to determine separate cytokine profiles for each infected virus and identify the ion channels and synaptic neurotransmitters associated with the identified cytokines.
3. Severe acute respiratory syndrome coronavirus 2
After the first report of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in December 2019 in China [47], the worldwide COVID-19 pandemic continues. COVID-19 is primarily a respiratory tract infection; therefore, FS is expected to occur in children infected with SARS-CoV-2 as with other respiratory viruses. A recent meta-analysis reported that fatigue or myalgia (18%) was most common among neurologic manifestations in children and adolescents with COVID-19, followed by smell or taste impairment (13%), headache (10%), and seizures (4%) [48]. FS occurred in 1.1%–2.3% of children with COVID-19 [49-51], a rate that was not higher than that in Korean children infected by influenza virus (5.0%) or by RSV (2.3%) [52,53]. SARS-CoV-2 is considered neurotropic, as are other coronaviruses, and neurons and glial cells express angiotensin-converting enzyme 2, the host receptor for SARS-CoV-2 [43,54]. Therefore, direct CNS invasion of SARS-CoV-2 may cause neurologic manifestations [54]. Moreover, indirect CNS damage triggered by post-infectious inflammatory responses, as in other respiratory viral infections, may also generate neurologic manifestations, including FS [54]. Recently, in Korea, the number of children with COVID-19, especially infants and young children who are vulnerable to FS, increased with the introduction of the Omicron variant [55]; therefore, an increase in children with FS accompanied by COVID-19 is expected. Considering that 13.6% of hospitalized children and adolescents with COVID-19 experienced FS in South Africa during the Omicron variant outbreak [56], neurologic manifestations including FS should be thoroughly monitored for in Korean children with COVID-19.
Genetic factors identified in febrile seizures
Among children with FS, 20%–30% have a family history of FS [7,33,57]; therefore, genetic factors are expected to play a role in FS. To date, genetic heterogeneity of familial FS (termed FEB1–FEB11) has been reported in various familial and linkage studies. Although several loci for familial FS have been verified, including FEB1 on chromosome 8q13-q21 (MIM number: 121210), FEB2 (602477) on chromosome 19p13, FEB3B (613863) on chromosome 2q24, FEB5 (609255) on chromosome 6q22-q24, FEB6 (609253) on chromosome 18p11, FEB7 (611515) on chromosome 21q22, FEB9 (611634) on chromosome 3p24.2p23, and FEB10 (612637) on chromosome 3q26, disease-associated genes have not been identified on them [58]. In contrast, genetic mutations were identified in FEB3A (604403), which is affected by SCN1A on chromosome 2q24; FEB4 (604352), which is affected by ADGRV1 on chromosome 5q14; FEB8 (607681), which is affected by GABRG2 on chromosome 5q31; and FEB11 (614418), which is affected by CPA on chromosome 8q13 (56, 58) [58-60].
GEFS+ (604233) is characterized by the onset of FS during infancy to early childhood and the subsequent development of various types of seizures [17]. GEFS+ is associated with several genetic mutations in ion channels, ligand-binding receptors, and proteins involved in synaptic transport, including SCN1A, GABRG2, GABRD, STX1B, and HCN2, as well as several chromosomal loci, including some on chromosome 2p24, 8p23-p21, and 6q16.3-q22.31 [17]. Dravet syndrome, one phenotype of GEFS+, is a rare genetic developmental and epileptic encephalopathy characterized by the infantile onset of intractable seizures that are often febrile as well as declining cognitive function [61]. Many patients with Dravet syndrome have SCN1A mutations [17,61], With regard to the later occurrence of epilepsy in children experiencing FS, some population-based association studies identified significant genes associated with both FS and epilepsy, including SCN1A, CHRNA4, GABRG2, and IL-Iβ [58,59].
The association between polymorphisms of genes encoding cytokines and FS has also been studied [59]. Although genetic polymorphisms of IL-1β, IL-1RA, IL-6, IL-10, and TNF-α were reportedly associated with FS in some studies, opposite results were also reported in other studies of these cytokines [59].
Genetic testing for children presenting with FS should not be performed routinely; however, pediatricians should carefully follow these patients and consider performing a genetic workup in children experiencing complex FS and subsequent afebrile seizures as well as in those with underlying neurodevelopmental disabilities.
Conclusion
The pathogenesis of FS is multifactorial and heterogeneous. There are no consistent and definite results regarding the connection between systemic or local inflammation and the CNS or on the mechanisms for increasing neuronal excitability in the CNS during fever. It is impossible to predict and prevent seizures in febrile children infected by a specific respiratory virus. However, life-threatening infections are very rare, and the underlying genetic disorders associated with subsequent epilepsy and neurodevelopmental disabilities are rare. In addition, the outcomes were generally good and not associated with the infected virus in children with FS. Therefore, intensive searches for the virus and vigorous neurologic and genetic evaluations are neither necessary nor helpful for affected patients.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.