Arrhythmia and COVID-19 in children
Article information
Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection caused worldwide health problems, and coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization in March 2020. Cardiovascular complications of COVID-19 are not uncommon; among them, arrhythmia is considered a significant risk factor for poor health outcomes in adults. However, data are scarce on the arrhythmia of pediatric patients with SARS-CoV-2 infection, possibly due to their mild symptoms and low incidence of cardiovascular involvement. Multisystem inflammatory syndrome in children reportedly features increased cardiovascular involvement, but arrhythmic complications remain unidentified. Thus, here we review the epidemiology, manifestations, and outcomes of pediatric arrhythmia associated with COVID-19.
Key message
· Pediatric patients have a relatively low incidence of tachyarrhythmia both in acute coronavirus disease 2019 and multisystem inflammatory syndrome in children (MIS-C), but it was associated with an increased risk of poor outcomes.
· Conduction abnormalities were not uncommon, especially in those with MIS-C. Most patients recovered to normal sinus rhythm; however, some progressed to advanced atrioventricular block and rarely required permanent pacemaker implantation.
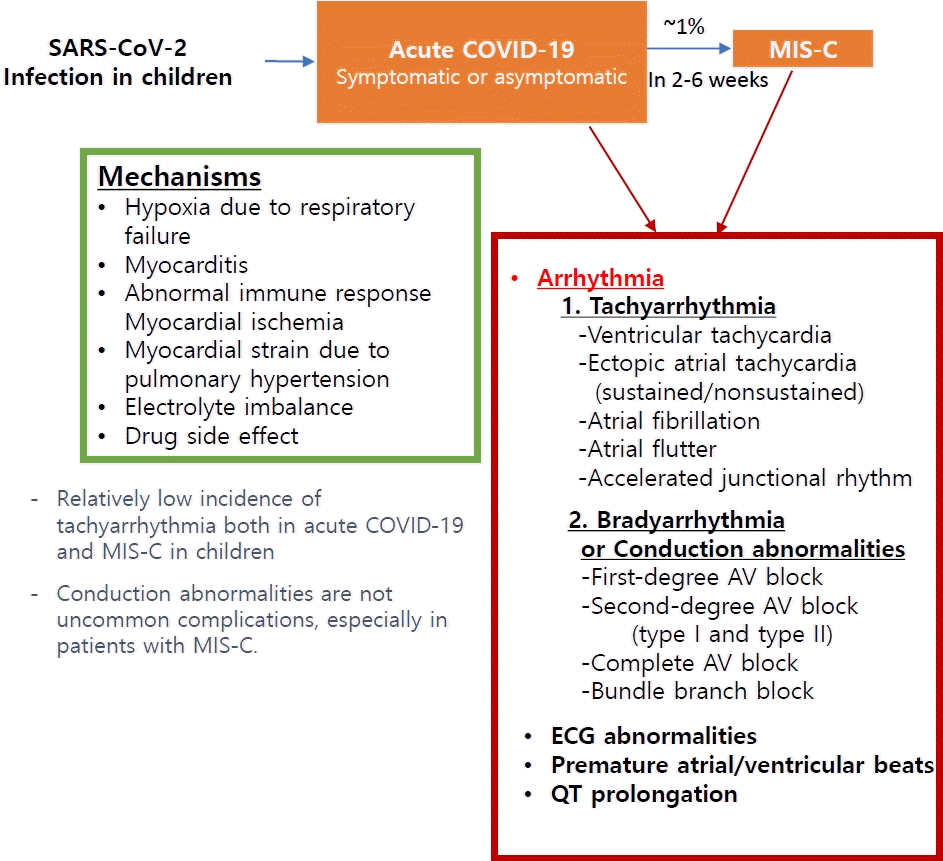
Graphical abstract. Arrhythmia associated with SARS-CoV-2 infection in pediatric patients. SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; COVID-19, coronavirus disease 2019; MIS-C, multisystem inflammatory syndrome in children; AV, atrioventricular; ECG, electrocardiogram.
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The rapid spread of the disease resulted in a global pandemic with 634 million confirmed cases and 6.6 million deaths reported as of late November 2022 by World Health Organization (WHO) [1]. Its clinical presentation ranges from asymptomatic to mild to severe respiratory illness, while its systemic complications include cardiovascular involvement. Its cardiovascular complications include acute myocarditis, pericarditis, vasculitis, heart failure, venous thromboembolism, and arrhythmia, all of which are associated with increased mortality in adults [2-5]. Children generally have milder symptoms than adults, but severe pediatric cases have been described [6]. Furthermore, multisystem inflammatory syndrome in children (MIS-C) with the feature of Kawasaki disease and toxic shock syndrome is a severe postinflammatory complication of SARS-CoV-2 infection reported mainly in children and adolescents [7-9]. Arrhythmia is among the critical cardiovascular complications reported in 18%–44% of adult patients [4,5,10,11]. However, there have been only a few reports of arrhythmia in pediatric patients, possibly related to the lower complication rates and milder disease course in this population. Therefore, the characterization, clinical course, and outcome of arrhythmia in the pediatric population have not been entirely clarified. This review aims to present the current state of knowledge and summarize the literature on arrhythmia associated with COVID-19 in pediatric patients.
Mechanism of cardiovascular complications of COVID-19
Coronaviruses are large and enveloped viruses with singlestranded RNA. While human coronaviruses cause common cold-like respiratory illnesses, SARS-CoV from China during 2002–2003 and Middle East respiratory syndrome coronavirus from the Middle East in 2012 and 2015 made outbreaks with increased fatality rates of 9.6% and 36%, respectively [12,13]. These viruses manifest not only mild respiratory symptoms but also severe illnesses such as acute respiratory distress syndrome and end organ damage.
SARS-CoV-2 utilizes human angiotensin-converting enzyme 2 (ACE2) as a host surface cellular receptor for virus entry similar to SARS-CoV.5,10) SARS-CoV-2 has a surface-located spike glycoprotein that includes a receptor-binding domain that is responsible for membrane fusion followed by endocytosis into target cells [14]. ACE2 expression is tissue-specific with distribution in the heart, intestines, kidneys, testes, and respiratory system, suggesting a correlation with extrapulmonary involvement [15,16]. Once the virus infects cells in the respiratory tract, viral replication and circulation can occur, leading to cardiac uptake and replication of the virus [17]. ACE2, together with ACE, plays a vital role in blood flow and volume via the renin-angiotensin system. ACE2 mRNA expression is upregulated in patients with heart failure, making those individuals more susceptible to SARS-CoV-2 infection and possibly explaining their worse outcomes [18].
The proposed mechanisms of myocardial injury include direct ACE2-mediated injury, hypoxia-induced injury from respiratory failure, microvascular thrombosis, and systemic inflammatory injury from an exaggerated immune response, including cytokine storm [19]. A reported 20%–30% of patients with COVID-19 showed elevated cardiac biomarkers such as troponin and B-type natriuretic peptide in the acute phase, suggesting that direct myocardial injury is not uncommon, especially in moderate to severely ill patients and non-survivors [3,20,21]. Elevated cardiac biomarkers are associated with COVID-19 severity and independent predictors of mortality. Cardiac magnetic resonance imaging has shown myocardial edema, impaired ventricular function, and myocardial fibrosis even after recovery from COVID-19 [19]. In cardiac autopsies, chamber dilatation, lymphocytic myocarditis, focal pericarditis, endocardial thrombosis, and small vessel thrombosis have been reported. Thus, SARS-CoV-2 may directly damage infected cardiac cells with inflammation, triggering severe cellular pathology and organ dysfunction [19].
Cardiovascular involvement of SARS-CoV-2 in children
Children infected with SARS-CoV-2 generally have mild symptoms, with reported mortality rates of <0.1% [6,22-25]. A significant number of children (15%–42%) are asymptomatic, whereas 18%–57% require hospitalization [25-29]. Typical symptoms and signs include fever (40%–64%), cough (33%–56%), fatigue, tachypnea, nasal congestion, feeding difficulty or intolerance, shortness of breath, sore throat, and headache [25-28]. However, severe cases requiring intensive care unit (ICU) admission have also been reported. In a European multicenter study of 582 individuals <18 years of age with polymerase chain reaction (PCR)-confirmed COVID-19, 8% of study participants required ICU admission, 4% required mechanical ventilation, 3% required inotropic support, and 1% required extracorporeal membrane oxygenation (ECMO) support [26]. A meta-analysis by Sumner et al. [6] demonstrated that, among 973 hospitalized school-aged patients, 10.1% required ICU admission, 4.2% had a severe outcome, and 1.1% (95% confidence interval, 0.2%–2.3%) died.
Cardiovascular involvement in children can occur in the setting of acute SARS-CoV-2 infection and post-infectious MIS-C [30]. During acute COVID-19 in children, cardiovascular complications are rare. Only a few case reports or case series have reported myocarditis, pericarditis, cardiogenic shock, and arrhythmia [31-33].
Cardiovascular involvement in children with MIS-C
MIS-C is characterized by hyperinflammatory shock syndrome with multiorgan involvement in previously asymptomatic children with SARS-CoV-2 infection and was first reported in case series in the United Kingdom in April 2020. It showed similar features of atypical Kawasaki disease, Kawasaki disease shock syndrome, or toxic shock syndrome [7]. Subsequently, similar cases began to be reported in series [34-36]. The United States Centers for Disease Control and the WHO termed this syndrome MIS-C [37,38]. It is also called pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 [39]. Among the pediatric patients with PCR-confirmed SARS-CoV-2 infection in an Australian multicenter study, 1.3% had MIS-C.
MIS-C reportedly occurs 2–6 weeks after COVID-19 symptom onset or contact with infected individuals [35,40-42]. Cardiac involvement in MIS-C occurs in up to 80% of patients [42]. Additionally, cardiogenic or vasodilatory shock is present in 37%–60% of MIS-C patients [9,43-46]. Half of one population of patients had reduced left ventricular (LV) ejection fraction on echocardiography, while 9%–17% developed coronary artery dilatation or aneurysm [9,35,44,46]. Myocarditis, valvular regurgitation, and pericardial effusion have also been identified. Arrhythmias or electrocardiographic abnormalities are common, reported in up to 60% of patients [35,47-49]. Outcomes are generally positive, with a resolution of inflammatory and cardiac abnormalities within 1–4 weeks. However, 0.3%–5% of patients received ECMO support, while 0%–2% of patients, most of whom were previously healthy individuals, died [9,35,43,46,48,50].
Arrhythmia in children with COVID-19 or MIS-C
Arrhythmia, reported in 18%–44% of adult patients with COVID-19, is associated with worse clinical health outcomes [4,5,10,11,51]. Among them, the majority (82%) developed atrial arrhythmias, 21% developed ventricular arrhythmias, and 23% had bradyarrhythmia [11]. Potential mechanisms of arrhythmia are hypoxia due to direct viral involvement in the lung, myocarditis, an abnormal host immune response, myocardial ischemia, myocardial strain due to pulmonary hypertension, electrolyte derangements, intravascular volume imbalances, and drugrelated side effects [52]. Arrhythmias are likely not only a direct effect of viral infection but also a complication of systemic illness [51].
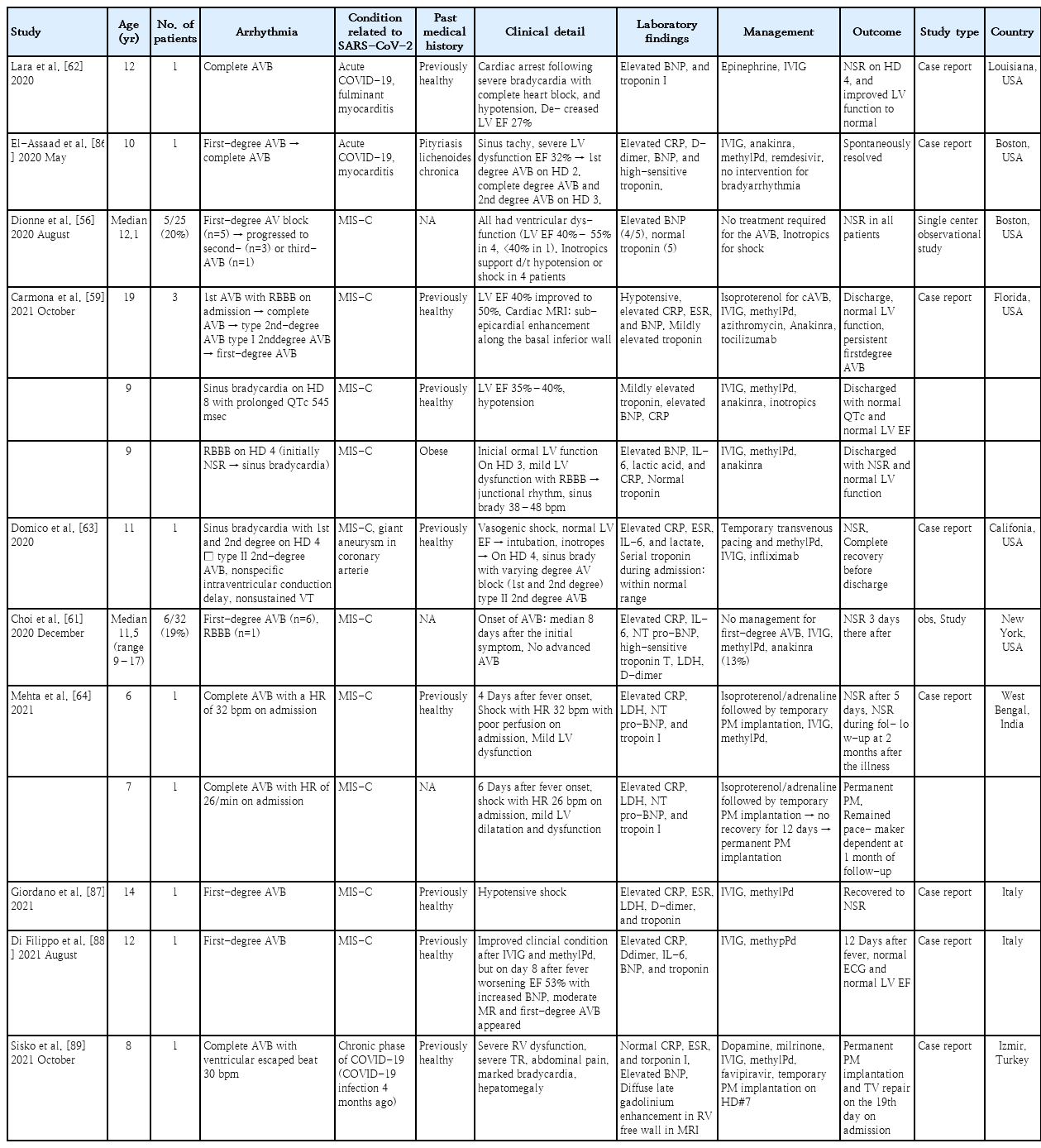
The incidence of arrhythmia associated with SARS-CoV-2 in children is much lower, with only a few case series or case reports to date. Tachyarrhythmia in pediatric patients was reported at a rate of 0%–17%, albeit some studies did not specify the type of arrhythmia and included isolated premature ventricular/atrial complexes [35,44,47,48,53,54]. On the other hand, multicenter studies reported the incidence of tachyarrhythmia as 1.7%–1.8%4 [3,55]. Variations in the incidence of arrhythmia may exist among studies because measures depend on the included patient population, such as hospitalized patients, public surveillance, or PCR-confirmed SARS-CoV-2 patients. Moreover, monitoring methods, such as 12-lead electrocardiography and continuous telemetry, may influence the variations. Published articles on tachyarrhythmia in children are summarized in Table 1; these include only those that demonstrated specified types of arrhythmia as well as their management, clinical details, and outcomes. Data for the patients with isolated premature atrial or ventricular complex and studies lacking descriptions of specific types of arrhythmia were omitted.
Samuel et al. [54] reported that 6 patients (17%) among a sample of 36 hospitalized pediatric patients with acute COVID-19 had tachyarrhythmia on continuous telemetry; 5 had nonsustained monomorphic ventricular tachycardia (VT), and one had sustained atrial tachycardia. None had bradyarrhythmia. Among the 6 patients, 4 had underlying disease such as sickle cell anemia, hematologic malignancy, and Bloom syndrome. Two had mild to moderate LV dysfunction and 4 had normal LV systolic function. All arrhythmias were self-limiting, but amiodarone in one and beta-blockers in 2 patients were used for prophylactic purposes.
Most of the reported cases showed a benign disease course or complete recovery with timely and aggressive treatment. However, mortality in pediatric patients with tachyarrhythmia has been reported, and large multicenter studies demonstrated that tachyarrhythmia was associated with poor outcomes [56-58]. Using a public health surveillance registry of 63 hospitals in the United States, Dionne et al. [55] showed the characteristics and outcomes of tachyarrhythmia in patients aged <21 years and hospitalized with acute COVID-19 or MIS-C. Twenty-two of 1,257 patients (1.8%) with acute COVID-19 and 41 of 2,343 patients (1.7%) with MIS-C had tachyarrhythmia, including supra VT (44%), accelerated junctional rhythm (14%), and VT (60%). Among them, 8 (13%) required cardiopulmonary resuscitation, while 9 (14%) required ECMO support because of refractory tachyarrhythmia. Patients with tachyarrhythmia were older and more frequently required mechanical ventilation and ECMO support. They also had higher illness severity on hospital admission, a longer hospital length of stay, and higher mortality rates (14% vs. 2%, P<0.001). Among the patients with tachyarrhythmia, those with acute COVID-19 showed higher mortality rates than those with MIS-C (7 of 22 [32%] vs. 2 of 41 [5%], P=0.006). Although tachyarrhythmia rarely develops in pediatric patients based on the large population data, it may have a substantial clinical impact and require close monitoring and aggressive treatment. Arrhythmia can increase an individual’s risk of death by deteriorating their clinical condition, while tachyarrhythmia can reflect a severe underlying cardiac and respiratory condition.
Conduction abnormalities associated with SARS-CoV-2 (e.g., first-degree atrioventricular [AV] block, second-degree AV block, complete AV block, sinus bradycardia, and bundle branch block) are more common complications than tachyarrhythmia in children. Table 2 shows the clinical characteristics, management, and outcomes of the patients reported in the literature. Bradyarrhythmia was reported in 16%–20% of patients with MIS-C [56,59,60]. MIS-C usually presents several weeks after a viral infection, and a dysregulated inflammatory response is the presumed pathophysiology [41]. Therefore, inflammation of conduction tissue and edema adjacent to the AV nodal or His-Purkinje system can be assumed to contribute to the conduction abnormalities. Coronary insufficiency to the AV node and other conduction systems is also a possible mechanism. Conduction abnormalities such as sinus bradycardia and bundle branch block reportedly developed after the administration of anti-inflammatory drugs during admission in some patients, suggesting drug-related bradyarrhythmia as one etiology [59].
First-degree AV block was the most common bradyarrhythmia [56,60,61]. It presented at a median 6–8 days after symptom onset, and most patients returned to a normal sinus rhythm spontaneously. A transient complete AV block or second-degree AV block (type I or type II) has been also reported, and some patients required temporary pacemaker insertion [44,56,62]. The incidence of a complete AV block was as low as 2.1%–5%, and most cases recovered a normal sinus rhythm [44,56]. However, some patients who developed a first-degree AV block progressed to a second- or third-degree AV block [56,59,63]. In rare instances, some children required permanent pacemaker implantation, and the first-degree AV block persisted at the time of discharge in some, suggesting the possibility of irreversible damage to the conduction tissue [59,64]. Therefore, patients with conduction abnormalities require intensive electrocardiogram monitoring during hospitalization and long-term follow-up after discharge [56,60,61]. The therapeutic effect of anti-inflammatory drugs on AV block or the preventive effect on the progression to a complete AV block remains unknown because all related studies were retrospective.
Electrocardiographic changes such as nonspecific T wave changes, low QRS amplitudes, an abnormal QRS axis, ventricular hypertrophy, and significant ST-segment changes were findings in 26%–35% of patients with acute COVID-19 and 35%–60% of those with MIS-C [43,44,49,54,58,65].
Repolarization abnormality
There have been reports of QT prolongation or repolarization abnormalities in patients with COVID-19 and MIS-C irrespective of QT-prolonging drug use [44,59,61,65-68]. Repolarization abnormalities, with myocardial injury from acute myocarditis in COVID-19 and a hyperinflammatory state in MIS-C, can increase the risk of malignant arrhythmia in patients with inherited arrhythmia despite no reports to date. Pawar et al. [69] reported neonates with multiple inflammatory syndromes presenting with QTc prolongation with a 2:1 AV block who were born to mothers with a history of COVID-19. An AV block with 2:1 conduction was a functional block due to QT prolongation and disappeared with intravenous immunoglobulin and methylprednisolone, followed by normalization of the QTc interval in all patients.
Furthermore, some drugs used in patients with COVID-19, such as remdesivir, azithromycin, and hydroxychloroquine, can prolong the QTc interval even in patients without underlying disease (www.crediblemeds.org) [38]. An electrolyte imbalance (hypokalemia, hypocalcemia, and hypomagnesemia) and dehydration from a poor oral intake, diarrhea, or vomiting may further increase the QTc interval [52]. So, patients with COVID-19 and an increased risk of QT prolongation require electrocardiography and QTc change monitoring during the illness. As patients with congenital long QT syndrome have an extended QT interval with the increased risk of torsades de pointes, they require electrocardiography monitoring during acute COVID-19 or MIS-C and must avoid QT-prolonging drugs and hypokalemia with COVID-19-associated diarrhea.
Autonomic dysfunction
Palpitations, dizziness, and orthostatic intolerance have been noted in some patients for weeks to months after the initial SARS-CoV-2 infection [70-72]. Postural orthostatic tachycardia syndrome and autonomic dysfunction are among the causes of such symptoms. The proposed mechanisms are dehydration, increased cardiac sympathetic outflow from damaged or altered autonomic nervous system function, and autoimmunity [71]. Although the mechanism is not yet clarified, the symptoms usually significantly improved with lifestyle modifications and medications such as fludrocortisone, midodrine, or beta-blockers [70].
Cardiovascular complications including arrhythmia in patients with congenital heart disease
Patients with underlying cardiac diseases, such as congenital heart disease (CHD) and cardiomyopathy, were initially considered at high risk for mortality and poor outcomes [57,73,74]. Strah et al. [75] demonstrated that pediatric patients with COVID-19 and moderate to severe CHD were younger at admission (1 vs. 11 years), had a longer length of stay, and had higher morbidity and mortality rates than those without CHD. However, other studies exhibited that worse New York Heart Association Functional class ≥III, genetic syndrome, and adults with CHD were significantly associated with the need for hospitalization/respiratory support as well as higher morbidity and mortality rates rather than CHD presence or severity [76,77]. A multicenter study from 58 adult CHD centers that included 1,044 infected patients also demonstrated that the COVID-19 case/fatality rate of 2.3% was similar to that (2.2%) of the general population. Cyanosis, previous heart failure admission, a worse physiological stage, pulmonary arterial hypertension, male sex, renal insufficiency, and diabetes were associated with death, while anatomic complexity was not predictive [78].
Patients with CHD are at increased risk for arrhythmia, but only one case series of arrhythmic events was published during the COVID-19 pandemic [57]. Simpson et al. presented 6 patients with CHD and one patient with hypertrophic cardiomyopathy for acute COVID-19, of whom 2 had newly developed VT requiring cardiopulmonary resuscitation or defibrillation. Both had respiratory failure and decompensated heart failure before the development of VT, and one eventually died of VT recurrence. Although the association between arrhythmia development in CHD patients and COVID-19 has not been identified, a proarrhythmic substrate in repaired or unrepaired CHD in a patient with diastolic and/or systolic dysfunction combined with right ventricular failure from respiratory failure may result in poor outcomes.
Conclusion
Cardiovascular complications are uncommon in children with SARS-CoV-2 infection, whereas those with MIS-C had increased cardiovascular involvement. Tachyarrhythmia was associated with poor clinical outcomes in a large multicenter study. Many patients improved with/without medical treatment, but some required ECMO support. Bradyarrhythmia was relatively common, reported in up to 20% of patients, and more frequently occurred in patients with MIS-C than acute COVID-19. Most patients with conduction abnormalities recovered a normal sinus rhythm, and permanent pacemaker implantation was rarely required.
Pediatric patients have a relatively low incidence of arrhythmic complications, but those with arrhythmia require a timely diagnosis with immediate aggressive treatment. Electrocardiography abnormalities and QT prolongation were not uncommon. Thus, among pediatric patients with acute COVID-19 or MIS-C, especially those who are critically ill, close monitoring for arrhythmia and QT prolongation is required as appropriate management. There are limited data for cardiovascular involvement in pediatric patients, and the effects of SARS-CoV-2 infection are still emerging. Therefore, continuous research with long-term follow-up is required.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.