Safety monitoring of COVID-19 vaccines: February 26, 2021, To June 4, 2022, Republic of Korea
Article information
Abstract
As of June 2022, 5 coronavirus disease 2019 (COVID-19) vaccine brands have been used in Korea’s national immunization program. The Korea Disease Control and Prevention Agency has enhanced vaccine safety monitoring through a passive web-based reporting system and active text message-based monitoring. In this study, an enhanced safety monitoring system for COVID-19 vaccines is described and the frequencies and types of adverse events (AEs) associated with the 5 COVID-19 vaccine brands were analyzed. AE reports from the web-based COVID-19 Vaccination Management System and text message-based reports from recipients were analyzed. AEs were classified as nonserious or serious (e.g., death or anaphylaxis). The AE reporting rates were calculated based on the number of COVID-19 vaccine doses administered. A total of 125,107,883 doses were administered in Korea from February 26, 2021, to June 4, 2022. Among them, 471,068 AEs were reported, of which 96.1% were nonserious and 3.9% were serious. Among the 72,609 participants in the text message-based AE monitoring process, a higher AE rate of local and systemic reactions was reported for the 3rd versus 1st doses. A total of 874 cases of anaphylaxis (7.0 per 1,000,000 doses), 4 cases of thrombocytopenia syndrome (TTS), 511 cases of myocarditis (4.1 per 1,000,000 doses), and 210 cases of pericarditis (1.7 per 1,000,000 doses) were confirmed. Six fatalities were causally associated with COVID-19 vaccination (1 of TTS and 5 of myocarditis). Young adult age and female sex were related with a higher AE rate for COVID-19 vaccines. Most reported AEs were nonserious and of mild intensity.
Key message
· Enhanced safety monitoring system of coronavirus disease 2019 (COVID-19) vaccines were implemented to detect signals rapidly as part of the national COVID-19 vaccination program.
· As of June 4, 2023, reported adverse events after COVID-19 vaccination was 0.38% among 125,107,883 doses of COVID-19 vaccines administered.
· Most reported adverse reactions after COVID-19 vaccinations have shown nonserious and mild intensity.
Introduction
As of June 4, 2022, 5 coronavirus disease 2019 (COVID-19) vaccines have been recommended against the COVID-19 since the first vaccine was rolled out on February 26, 2021 in the Republic of Korea (Korea) under the approval of the Ministry of Food and Drug Safety (MFDS) [1,2]; the AstraZeneca COVID-19 Vaccine (ChAdOx1-S, hereafter “AstraZeneca vaccine”), and the Janssen COVID-19 Vaccine (Ad26.COV2.S, hereafter “Janssen vaccine”)—viral vector vaccines; the Pfizer-BioNTech COVID-19 vaccine (BNT162b2, hereafter “Pfizer vaccine”), and the Moderna COVID-19 Vaccine (mRNA-1273, hereafter “Moderna vaccine”)— mRNA based vaccines, and Nuvaxovid prefilled syringe COVID-19 Vaccine (NVX-CoV2373, hereafter “Novavax vaccine”)—protein subunit vaccine.
The national vaccination plan was designed with the Korea Advisory Committee on Immunization Practices’ recommendation based on recipient age, epidemiologic situation and vaccine supply [3,4]. The highest priority for vaccination at the beginning of 2021 included healthcare personnel in frequent close contact with COVID-19 patients, residents and staff at long-term care facilities, and older adults. The recommendation was expanded to all eligible people with vaccine availability, and revised according to new scientific knowledge including adverse events (AEs) and epidemiologic status; Pfizer vaccine for individuals aged 5 years and older; Moderna and Janssen vaccine for those aged 30 years and older; AstraZeneca vaccine for those aged 50 years and older, and Novavax vaccine for adults (≥18 years). A primary series of 2 doses for COVID-19 vaccines except a single dose of Janssen COVID-19 vaccine were recommended with interval of 3 weeks to 12 weeks depending on vaccine brand, supply and epidemic situations. After completing their primary series, booster has been recommended; 1 booster (3rd dose, 3 months after the 2nd dose) for everyone aged 18 years and older, and eligible adolescents aged 12–17 years, and 2 boosters (4th dose, 4 months after the 3rd dose) for high-risk groups, such as immunocompromised individuals, and older adults (≥60 years). People received Janssen vaccine as their first dose have been recommended their booster dose 2 months after the initial dose. As of June 4, 2022, a total of 125,107,883 doses of COVID-19 vaccines were administered in Korea, of which 33,248,513 were third doses; 70.8% of the adult population (≥12 years) has received 3 doses, and 95.6% of aged over 12 years have received at least 1 dose of COVID-19 vaccine (Supplementary Fig. 1) [5].
As vaccination initiated, passive and active safety monitoring measures were implemented for rapid signal detection of safety issues associated with COVID-19 vaccines; First, vaccine recipients or their caregivers can use the COVID-19 vaccination assistant website (www.ncv.kdca.go.kr) for direct AEs reporting. Second, health problems and disturbances in everyday life were monitored through text messages sent to all vaccine recipients, and for vulnerable vaccine recipient groups, such as children, adolescents, and pregnant women, active monitoring was conducted through text messages by the concerned vaccine brand during the initial phase of vaccination. Third, in the event of serious AEs—that is, hospitalization, disability, and death—and AEs of special interest (AESIs), rapid epidemiological investigations were conducted to evaluate their causal association with the vaccine.
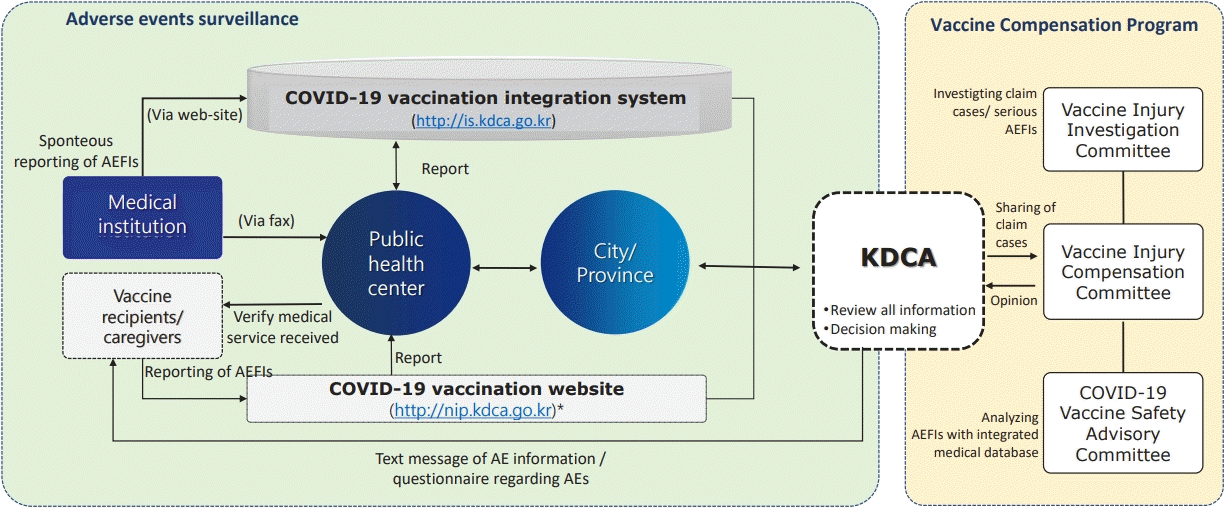
Among the reported AEs, symptoms such as pain at the injection site, headache and fever were classified as nonserious AEs; death, anaphylaxis, and AESI as serious AEs. Among serious AEs, those classified as severe cases include intensive care unit (ICU) admission, disability, life-threatening conditions, congenital anomaly, and death [6]. Reported AEs data were analyzed and calculated based on the information provided by healthcare providers when reporting suspected AEs following COVID-19 vaccination, whereby medical record review or diagnostic accuracy of each reported as AEs was not verified. The COVID19 safety monitoring measures implemented are described in Fig. 1. In this study we analyzed passive and active monitoring data of AEs following COVID-19 vaccination from 26 February, 2021, at the time of first COVID-19 vaccine rolled out to June 4, 2022. Subgroup analysis was conducted according to age, sex and vaccine brand.
Methods
1. AE monitoring system by healthcare personnel under the Infectious Disease Control and Prevention Act
Article 11 of the Infectious Disease Control and Prevention Act stipulates that medical doctors or forensic pathologists who “diagnoses a person indicating AEs to a vaccination, or examines the corpse of such person” are required to report the case via the web-based COVID-19 vaccination management system (http://is.kdca.go.kr). The AE reporting requires information such as vaccinated individual, vaccine brand, and AEs concerned after each dose of COVID-19 vaccination.
2. Self-report AE monitoring system
As a strategy to strengthen COVID-19 vaccine safety monitoring, the reporting system received self-report AEs via the COVID-19 vaccination website (https://nip.kdca.go.kr) where vaccine recipients or their caregivers can report any suspected symptoms after COVID-19 vaccination, then the competent public health center verifies corresponding medical treatment data and performs AE reporting to the COVID-19 vaccination management system.
3. Text message-based AE monitoring system
Text message surveys were conducted to implement an active safety monitoring of COVID-19 vaccines. A text message of mobile phone was sent to all vaccine recipients 3 days after vaccination. For the virus vector vaccine, text messages were sent 3 times on days 3, 7, and 14 after vaccination. Vulnerable population groups, such as children, adolescents, and pregnant women, were monitored daily from day 0 to day 7 using the text message survey. Survey questionnaires included health problems (Y/N), AE severity and symptoms, disturbances in everyday life (Y/N), and visit to a healthcare provider (Y/N). The results from text message survey were published in another journal [7]. Additionally, children and adolescents were monitored on the day of 6th week after the second dose. In the survey, the recipient was required to follow the URL embedded in the text message and answer questions about AE symptoms (Y/N) and hospital visit (Y/N) following which individual was given guidance regarding the AE concerned.
4. Eligibility and causality assessment
In Korea, causality assessment of AEs following immunization has been conducted by the Vaccine Injury Investigation Committee (VIIC) and the Vaccine Injury Compensation Committee (Fig. 1). The VIIC conducted prompt epidemiological investigation for serious AEs cases (e.g., death and ICU admission) including AESIs, and reviewed the causal association between AEs and COVID-19 vaccines. Reported AESI cases including anaphylaxis, myocarditis, pericarditis, thrombosis with thrombocytopenia syndrome (TTS), Guillain-Barré syndrome and idiopathic thrombocytopenic purpura were reviewed for eligibility diagnosis based on the Brighton collaboration definition using the diagnostic flowchart mapped by the expert advisory groups. Regarding AEs following COVID-19 vaccination, the COVID-19 Vaccine Safety Committee provides an evidence-based causality assessment framework based on the analysis of big data including nationwide AEs report data and related medical insurance data.
5. Data analysis
Descriptive analyses were applied for the reported AEs from the web-based system and text message-based survey and presented the results as frequencies and percentages. We did not exclude duplicated cases between the web-based COVID-19 vaccination management system and the text message-based reporting. Although the target age group differed for each vaccine, analysis was performed without its adjustment. Those who reported symptoms at least once during 7 consecutive days were considered symptomatic. SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Results
1. AE reporting status from the web-based COVID-19 6404 vaccination management system
A total of 471,068 AE cases after COVID-19 vaccination were reported in the COVID-19 vaccination management system during the study period (February 26, 2021 to June 4, 2022). Among them, 96.1% (452,530 cases) were nonserious AEs, and 3.9% (18,538 cases) were serious AEs; of the latter, 1,654 cases (0.4%) were fatal cases. By vaccine brand, 240,126 cases (51.0%) were reported after Pfizer vaccination; 111,471 (23.7%) after Moderna vaccination; 109,809 (23.3%) after AstraZeneca vaccination; 8,855 (1.9%) after Janssen vaccination, and 807 (0.2%) after Novavax vaccination. Vaccination rates for adult males and females (≥18 years) were similar, but females’ AE reporting rate was 1.8-fold higher than that of males. In terms of age group, reporting rate was highest in the 18–39 years, and decreased with increase in age. By dose, dose 1 resulted in the highest reporting rate, and the virus vector vaccine was associated with a high AE reporting rate (Table 1).
Vaccine types used for the booster dose (3rd dose) for adults (≥18 years) were, for the most part, mRNA vaccines (Pfizer vaccine and Moderna vaccine) or Novavax vaccines. AEs reported following the booster dose until June 4, 2022 totaled 54,140 cases (162.8 cases per 100,000 doses), with females outnumbering males by 1.5 times. Most of the reports were nonserious AEs (51,858 cases, 95.8%), and the most frequently reported AE was myalgia (39.8 cases per 100,000 doses), followed by headache (35.0 cases per 100,000), chest pain (27.8 cases per 100,000 doses), dizziness (21.0 cases per 100,000 doses), and nausea (18.2 cases per 100,000). The Pfizer vaccine was recommended as a booster dose for adolescents (12–17 years) who completed a primary series of 2 doses, and 1,056 AE cases (254.1 cases per 100,000 doses) were reported after a booster dose. Unlike in adults, the sex ratio was balanced: 1.1 times in adolescents and 1.5 times in adults in favor of females. Most of the reported cases were nonserious AEs (1,042 cases, 98.7%), and the most frequently reported AE was headache (93.7 cases per 100,000 doses), followed by muscle pain (79.7 cases per 100,000 doses), fever (58.5 cases per 100,000 doses), chest pain (45.0 cases per 100,000 doses), and dizziness (35.9 cases per 100,000 doses) (Table 2, Fig. 2).

Reported adverse events (AEs) after booster (dose 3) vaccination, February 26, 2021, to June 4, 2022
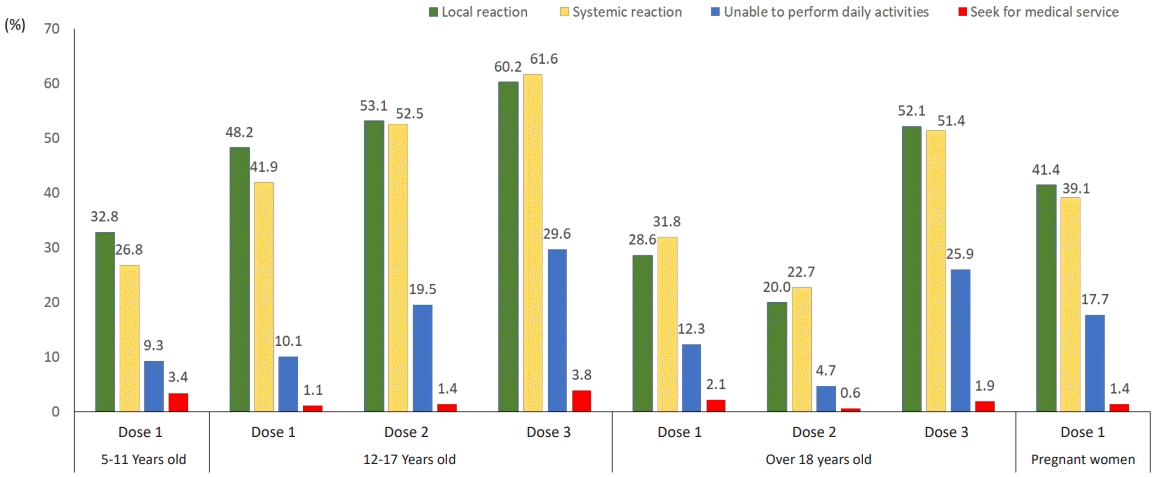
Self-reported adverse events among respondents within one week of coronavirus disease 2019 vaccination. Values represent the percentages of respondents who reported adverse events at least once during the days of text message survey (0–7 days after COVID-19 vaccination). Local reactions included injection site reactions, pain, swelling, redness, itching, and urticaria. Systemic reactions included fever, chills, arthralgia, fatigue, nausea, vomiting, headache, myalgia, armpit pain, diarrhea, abdominal pain, and rashes. The results of the first and second doses at 5–11 and 12–17 years were adapted from a previous study [21].
2. Results of text message-based AE monitoring in vaccine recipients
Text message-based AEs monitoring in the general adult population led to the following findings: most respondents reported local reactions after the 3rd dose compared to after the initial 2 doses (dose 1: 28.6%, dose 2: 20.0%, dose 3: 52.1%). Similar observations were found regarding systemic reactions (dose 1: 31.8%, dose 2; 22.7%, dose 3:51.4%). In the survey of adolescents (12–17 years) as well, more AEs were reported after the 3rd dose compared to the initial 2 doses for both local and systemic AEs (local AEs—dose 1: 48.2%, dose 2: 53.1%, dose 3:60.2%; systemic AEs—dose 1: 41.9%, dose 2: 52.5%, dose 3: 61.6%). Among children (5–11 years), local and systemic AEs accounted for 32.8% and 26.8%, respectively. Among pregnant women, 41.4% reported local AEs and 39.1% systemic AEs after the 1st dose (Fig. 2, Supplementary Table 1).
3. AEs of special interests
Reports of anaphylaxis, myocarditis, pericarditis, and TTS were reviewed by the local government’s rapid response team and the VIIC, confirmed anaphylaxis after COVID-19 vaccination occurred 7.0 per million doses, with a more than two-fold-higher prevalence in women compared to men (9.8 vs. 4.1) (Table 3). Of the anaphylaxis cases, 80.8% (706 cases) occurred within 30 minutes and 59.0% (565 cases) within 15 minutes; all cases were recovered after treatment, with no deaths reported.
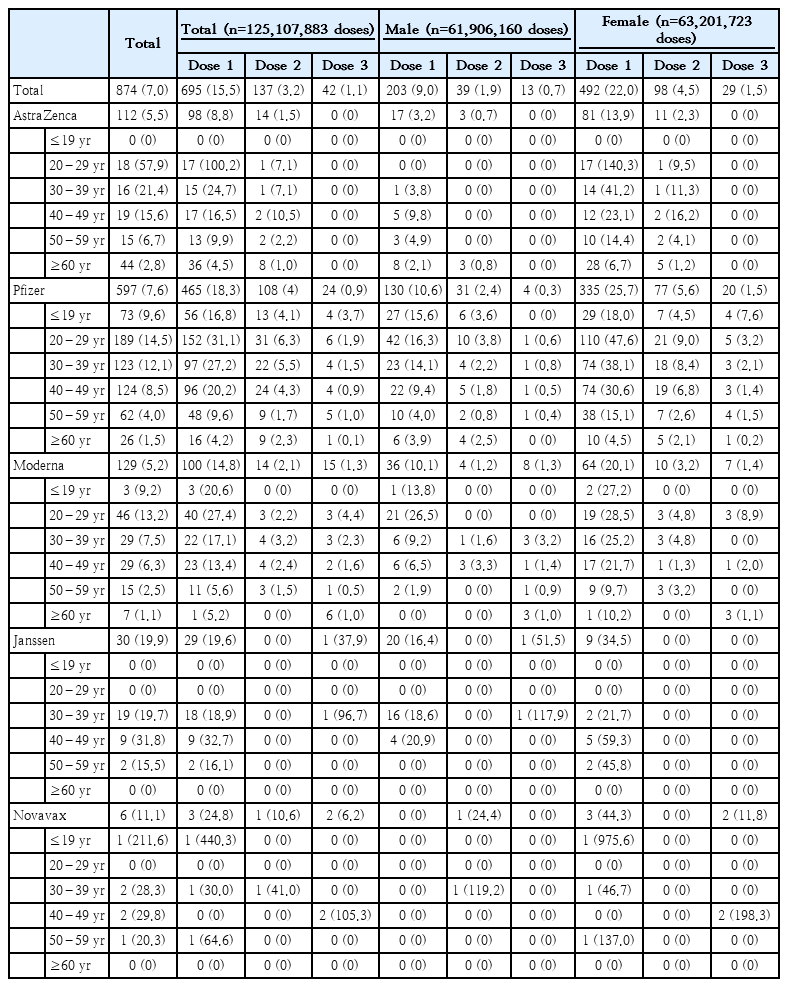
Confirmed anaphylaxis cases based on Brighton collaboration definition after coronavirus disease 2019 (COVID-19) vaccination, February 26, 2021, to June 4, 2022
In the case of suspected TTS, diagnostic testing was performed including the PF4 enzyme-linked immunosorbent assay antibody test, and the hematology advisory committee for COVID-19 vaccination reviewed all suspected cases and confirmed diagnosis. As of June 4, 2022, 4 cases (3 males; 1 female) met the diagnostic criteria for TTS; all 4 cases were associated with AstraZeneca vaccine (0.2 cases per million AstraZeneca vaccine doses). Three recovered after treatment, but 1 died. So far, 1,614 cases of suspected myocarditis and pericarditis were reported. The cardiology advisory committee for COVID-19 vaccination reviewed medical records to assess diagnostic accuracy, which resulted in 4.1 cases of myocarditis and 1.7 cases of pericarditis per million doses. For both myocarditis and pericarditis, higher rates were observed after the Moderna vaccination compared to the Pfizer vaccination (Table 4).
Discussion
Vaccination is known for safe, cost-effective and critical means against infectious diseases prevention [8]. However, even when vaccines are administered appropriately, they can induce unintended and undesirable AEs—much like other pharmaceutical products, despite all COVID-19 vaccines demonstrated excellent safety and clinical efficacy profiles in clinical trials and marketing approval by the MFDS. AEs do not necessarily have a causal relationship with the treatment [9]. If vaccination rate decreases because of excessive anxiety about AEs, mortality rate may increase because of increased spread and severity of vaccinepreventable diseases. After the COVID-19 pandemic, each country has implemented its COVID-19 vaccination program to prevent severe illness and death and to reduce transmission. The World Health Organization (WHO), the European Medicine Agency (EMA), and individual countries have released vaccination statistics and AEs monitoring results [10,11]. In Korea, in addition to the existing passive COVID-19 vaccination AEs monitoring, text message-based active AEs monitoring has been implemented. Additionally, for vaccine recipients reporting suspected myocarditis, pericarditis, or anaphylaxis after COVID19 vaccination, KDCA has recommended that those recipients be restricted from the same platform vaccine until the experts group reviewed their diagnosis.
In this study, the COVID-19 vaccination AEs reporting rate in Korea is 376.5 cases per 100,000 doses—slightly higher than that of the United Kingdom (UK) at 319.9 cases per 100,000 doses (141,355,000 doses; 452,155 AE reports) [12]. As similar to previous report, over 96% of AEs reports was nonserious AEs, which was also similar to that of the United States (US) (nonserious AEs following dose 3: 92.4%) [13,14]. The WHO and the EMA [12,15] have warned of TTS risk induced from the AstraZeneca vaccine. With those early warning, the Korea safety monitoring data supported to change the recommendation of COVID-19 vaccines and to focus special attention on AEs in Korea; AstraZeneca vaccines limited to the age group below 50 years, Janssen vaccines to over 30 years.
Based on the data released by the European Centre for Disease Prevention and Control and the UK’s Department for Business, Energy & Industrial Strategy, a study estimated the incidence of TTS among AstraZeneca vaccine dose 2 recipients at 2.3 per million, similar to the background incidence rate of the unvaccinated population [16]. In Korea, a lower incidence rate of TTS was estimated (0.2 cases per million doses), and among them, one fatal TTS case was associated with AstraZeneca vaccine. However, simple comparison has limitations, given the different target population groups and diagnostic accuracy standards among vaccine brands.
With some differences in reaction patterns among vaccine brands, incidences of anaphylaxis were positively associated with being young and female, as demonstrated in a US-based study [17]. Rate of anaphylaxis has been gradually lower after early phase of COVID-19 vaccination from 12.3 cases per million doses to 7.0 cases per million doses and no fatal case was reported [18].
Reports of incidence of myocarditis and pericarditis after Moderna vaccination were higher compared to after Pfizer vaccination, whereupon a few European countries restricted Moderna vaccination in the below-30 age group. Although no significant difference in the incidence of myocarditis and pericarditis was observed between Moderna and Pfizer vaccines in Korea, the Moderna vaccine was recommended for the above-30 age group as a preemptive measure. Among myocarditis reports following mRNA COVID-19 vaccination, 6 fatal cases were associated with vaccines in Korea [19].
There are some limitations to interpreting this data because the reported data used in this study are provisional statistics, with their reported diagnoses yet all unviewed. Furthermore, we did not distinguish duplicated AE reports between difference reporting methods. However, it is not likely to effect on estimation AE rate because AE reports by the text message-based survey or the self-the report system were relatively small portion of total AE reports. Given that the current AEs monitoring results mostly reflect short-term COVID-19 vaccine AEs, additional research is required for diagnostic accuracy assessment and long-term follow-up in tandem with continuous AE monitoring.
In conclusion, young adult and female sex were related with a higher reported rate of AEs following COVID-19 vaccination, most of reported AEs was nonserious AEs. AE reports were not different from previous clinical trials and other country’s reports.
Supplementary materials
Supplementary Table 1 and Fig. 1 can be found via https://doi.org/10.3345/cep.2023.00815.
Self-reported adverse events after COVID-19 vaccination based on a text-based survey
Initially recommended vaccine and start date for each group and vaccination rate as of April 30, 2022. AZ, AstraZeneca COVID-19 vaccine; Pf, Pfizer-BioNTech COVID-19 vaccine; M, Moderna COVID-19 vaccine; N, Novavax COVID-19 vaccine. Vaccination start date: AZ, 02/26/2021; Pf, 02/27/2021; J, 06/10/2021; M, 06/16/2021, N, 02/14/2022. Recommended for booster doses: people >18 years old who completed their primary doses. COVID-19, coronavirus disease 2019.
Booster dose administered at least 6 months after completion of primary series. However, the interval had been changed to 4-5 months as of Nov. 22, 2021, and was changed to 3 months as of Dec. 13, 2021. A 2-month interval is recommended for people who received Janssen vaccine as a primary dose or immunocompromised people.
People who received AZ vaccines as primary doses were recommended to receive mRNA vaccines (Pfizer or Moderna). People who received mRNA vaccines as primary doses were recommended to receive mRNA vaccines (Pfizer or Moderna). People who received Janssen vaccine as a primary dose were recommended to receive mRNA vaccines (Pfizer or Moderna). The Janssen booster dose was allowed for special occasions including prisoners. People 12–17 years of age were recommended (Pfizer or Moderna) only for high-risk groups such as immunocompromised people, and vaccination is recommended starting March 31, 2022. The COVID-19 vaccination rate as of June 4, 2022, was adapted from reference [22].
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: Lee YK, Kwon Y, Kim EK and Cho E; Data curation: Seo SY, Kim SY, Kim S, Ko M and Cho H; Formal analysis:Kwon Y, Heo Y, Kim SY, and Kim S; Methodology: Kwon Y, Kim EK, Seo SY, Kim SY, Lee YK, and Cho H; Project administration: Kwon Y, Lee YK, and Cho E; Visualization: Kim S, Ko M and Heo Y; Writing - original draft: Lee YK, Kwon Y, and Kim EK; Writing - review & editing: Lee YK, Kwon Y, Heo Y, Kim EK, Kim SY, Cho H, Kim S, Ko M, Lim D, Seo SY, Cho E
Acknowledgements
We thank all respondents in the text message-based survey after COVID-19 vaccination. We further thank staff in local public health centers, cities and provinces, and in private health facilities for their tireless efforts in monitoring adverse events after COVID-19 vaccination.