Skin and oral intervention for food allergy prevention based on dual allergen exposure hypothesis
Article information
Abstract
Early-onset atopic dermatitis increases an individual’s risk of food allergies, suggesting that transcutaneous sensitization may occur through inflamed skin. Regarding food allergy causation, the dual allergen exposure hypothesis proposes that oral allergen exposure leads to immune tolerance, whereas allergen exposure via inflamed skin causes food allergies. This hypothesis suggests that it is important to induce oral immune tolerance and prevent allergic food sensitization through the skin. This review focuses on the breakthrough evidence based on the dual allergen exposure hypothesis that involves both skin and oral interventions for food allergy prevention.
Key message
To prevent food allergy in infants, based on the dual allergen exposure hypothesis, we recommend a personalized approach consisting of both skin intervention (eczema treatment to achieve early remission and well-controlled skin without eczema to prevent percutaneous immunoglobulin E sensitization) and oral intervention (early allergenic food introduction).
Introduction
Food allergies and allergic diseases are widespread in South Korea, Japan, and other countries [1-4]. The emergence of food allergy epidemics has become a global concern, while its psychosocial and economic tolls have become a public health concern [5]. Food allergies affect approximately 10% of children in the United States [6] and Japan [7]. The major allergens responsible for food allergies vary among countries and ethnicities. Peanut allergies are the most common food allergies in North America and Europe, whereas hen’s egg allergies are prevalent in Australia and Asia [8].
Interest has recently increased in preventing immunoglobulin E (IgE)-mediated food allergies [9]. Although the mechanisms of sensitization and food allergy onset are not fully understood, a major shift in preventive approaches to food allergies is underway. It has been suggested that environmental and genetic factors (including epigenetic changes) may also influence the development of food allergies [10]. Factors that reportedly influence the risk of developing food allergies in childhood include genetic and environmental factors (family history [11], specific genes [12], atopic dermatitis [AD] [13], gut microbiota [14], sunlight, and vitamin D [15]). AD is a major risk factor for the onset of food allergies. Food allergy prevention is defined as the primary prevention of sensitization (production of specific IgE antibodies) and secondary prevention of the development of food allergies in sensitized individuals [16]. Lack [17] proposed the dual allergen exposure hypothesis, which states that percutaneous exposure of infants with eczema to food antigens induces an allergenic immune system that enhances allergy, whereas the oral intake of food antigens induces immune tolerance by enhancing the immune system that suppresses allergy. To prevent the onset of food allergies, it is essential to prevent transdermal sensitization by controlling AD and inducing oral immune tolerance by initiating oral intake during early infancy [18].
We understand that various environmental factors, such as delivery mode, animal exposure, air pollution, smoking, and medications such as antibiotics and harmful chemicals, might modify the allergy mechanism [19-22]. This review focuses on breakthrough evidence based on the dual allergen exposure hypothesis—which consists of both skin and oral interventions for food allergy prevention, excluding environmental interventions (Fig. 1).

Breakthrough evidence based on the dual allergen exposure hypothesis consisting of skin and oral interventions for preventing food allergies. IgE, immunoglobulin E; PACI, Prevention of Allergy via Cutaneous Intervention; JACI, Journal of Allergy and Clinical Immunology; NEJM, New England Journal of Medicine; LEAP, Learning Early About Peanut; PETIT, Prevention of Egg Allergy with Tiny Amount Intake; SPADE, Strategy for Prevention of Milk Allergy by Daily Ingestion of Infant Formula in Early Infancy.
AD is a significant risk factor in food allergy onset
Lack et al. [23] reported an association between eczema and peanut allergy, specifically severe dermatitis, as well as skincare containing peanut oil as risk factors for the onset of peanut disease, suggesting sensitization to percutaneous peanut allergen exposure. A cohort study in Australia reported that eczema in infancy increased the risk of developing peanut and hen egg allergies later in life [24]. We previously reported that the onset of eczema at 1–2 months of age was associated with the highest adjusted odds ratio (aOR, 6.61; 95% confidence interval [CI], 3.27–13.34), followed by 3–4 months of age (aOR, 3.90; 95% CI, 2.34–6.52) from a general birth cohort in Tokyo [25]. We also reported that persistent eczema was associated with food allergies at 3 years of age in a national birth cohort in Japan [13]. A Canadian birth cohort study demonstrated that AD at 1 year of age was associated with a higher risk of food allergies at 3 years of age [26]. There are several phenotypes of AD [27,28]. A European birth cohort showed that early-onset AD is a risk factor for food allergy [28]. According to these reports, AD is a major risk factor for food allergies. The earlier AD develops, the higher the risk of food allergy development.
Environmental food allergens
Environmental food allergens have been identified, including tables, beds, hands, and dust [29]. In Japan, we detected hen’s egg allergens in the bed dust of 3-year-old children and peanut and hen’s egg proteins in pet fur [30,31]. The concentration of hen’s egg protein in the dust in living rooms and bedrooms increases after the consumption of hen’s eggs at home [32]. In the United States, the levels of peanut protein in dust are higher at schools than at home [33]. In the United Kingdom, one study demonstrated a positive association between peanut antigen levels in dust, infant peanut sensitization, and peanut allergy [34]. Peanut allergy is associated with the reaction of T cells that express homing receptors on the skin to peanut antigens, suggesting a mechanism of percutaneous sensitization [35]. Food allergens are thought to contribute to food allergies in children via sensitization through inflamed skin, such as eczema.
Early enhanced anti-inflammatory treatment of eczema and maintenance of remission
For allergen exposure via inflamed skin, an animal study demonstrated that, when mice were exposed to peanut and ovalbumin allergens across disrupted skin,they developed a potent systemic immune response to them [36]. As previously stated, several cohort studies and a systematic review indicated that early-onset eczema is among the most important risk factors for food allergies [24,25,37-39]. We recently identified 1-month-old infants who had already developed AD as evidenced by mRNAs in their sebum [40]. As early interventions to prevent or treat AD, emollients (moisturizers) [41,42] or pimecrolimus using the reactive method alone did not prevent food allergies [43]. Emollients and weak anti-inflammatory medications applied only to clinically affected skin lesions may be insufficient for preventing food allergies. Emollients (moisturizers) are not anti-inflammatory products. In 2014, we conducted the first confirmatory randomized controlled trial (RCT) of AD prevention using moisturizer [44]. We discovered that applying moisturizers to infants with a family history of AD reduced the onset of AD, but it did not affect the onset of food allergies.
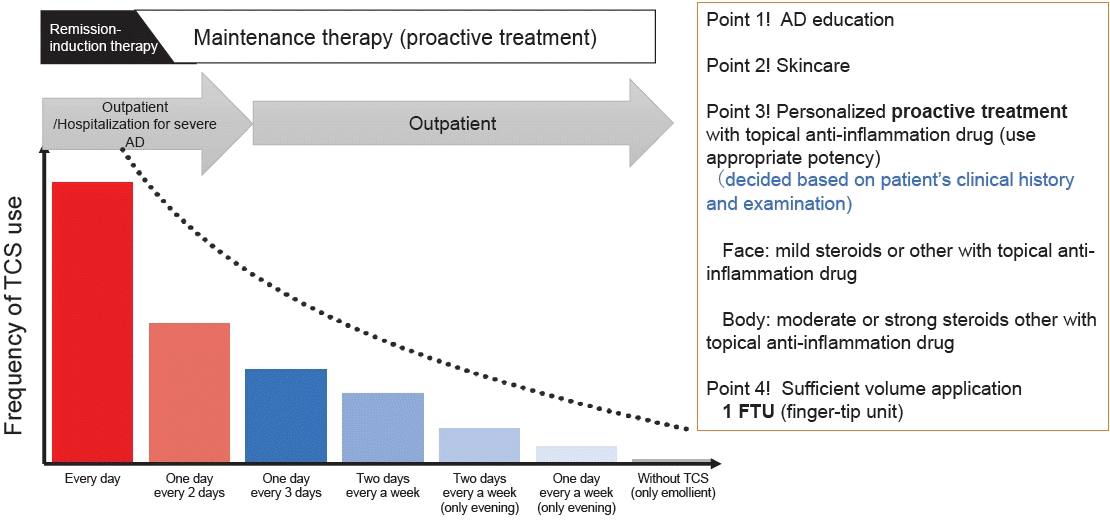
Guttman-Yassky et al. [45] presented strong evidence that immune and barrier abnormalities exist in both clinically affected and unaffected skin lesions in AD, suggesting that sufficient anti-inflammatory treatment of the whole body, including clinical and subclinical lesions, is required for food allergy prevention through the skin route. Proactive treatment (Figs. 2, 3) is a long-term maintenance approach that uses low-dose intermittent anti-inflammatory topical agents, such as topical corticosteroids, to treat and prevent flare-ups of chronic subclinical skin inflammation in AD [46-50]. We showed the proactive treatment protocol on a daily basis at our allergy center [51] (Figs. 3, 4). Approximately 90% of children with severe AD are able to achieve good control of their eczema (clear or mild AD) through proactive treatment and education from our real-world data. 51) Impaired skin barrier function and inflamed skin, such as in AD, have been suggested as risk factors for percutaneous sensitization to food allergens.

A case of severe atopic dermatitis treated with remission induction therapy and maintenance therapy (proactive therapy). AD, atopic dermatitis; TARC, thymus and activation-regulated chemokine. We obtained infromed consent for publication from her parent.
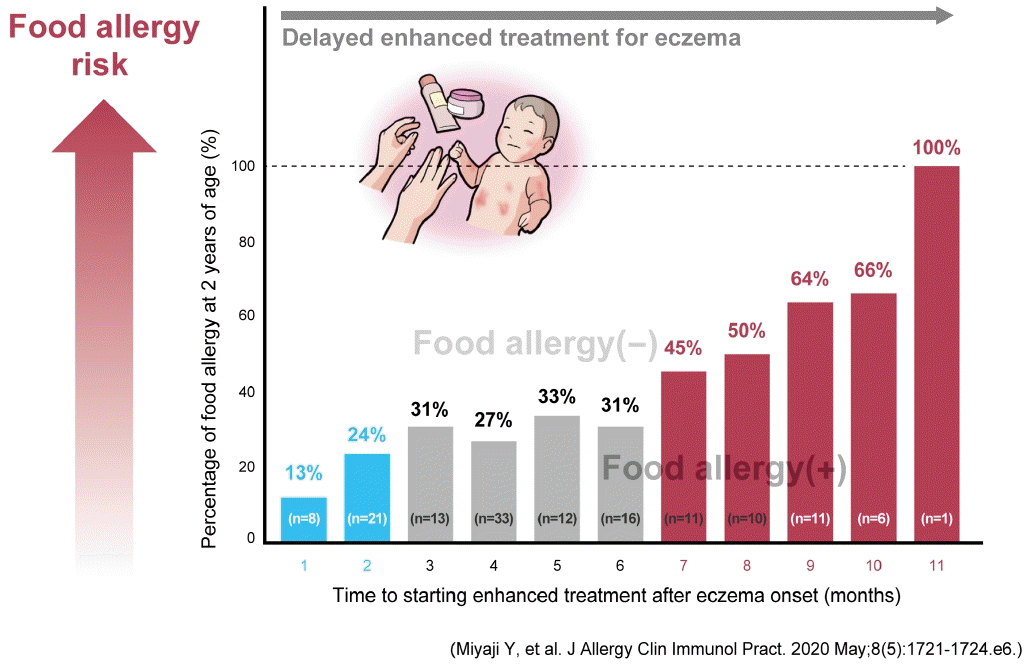
A case-control study by Yamasaki et al. [52] demonstrated that infants with AD who started proactive therapy by 4 months of age had a significantly lower incidence of hen’s egg allergy by 18 months of age versus those who started proactive therapy after 5 months of age (9.1% vs. 24.2%, respectively). We also found a significantly lower prevalence of food allergies at 2 years of age in the group of infants with AD who started proactive therapy within 4 months of developing AD versus those who received delayed treatment for AD (Fig. 5) [51]. These results suggest that, in children withAD, starting enhanced therapy for remission induction as early as possible and maintaining remission consisting of well-controlled skin may reduce the risk of developing food sensitization and food allergy by preventing percutaneous IgE sensitization to food allergens.
We recently completed the Prevention of Allergy via Cutaneous Intervention (PACI) study [53,54], a multicenter, investigator-blinded, randomized, and parallel group-controlled trial of an early enhanced intervention for infantile AD to prevent the onset of food allergy. Enhanced proactive treatment for infant AD reduces the risk of hen’s egg allergy, but with some concerns regarding early growth. The PACI study provides a new and convincing proof of concept that enhanced treatment of early-onset AD can reduce the risk of hen’s egg allergy onset based on the dual allergen exposure hypothesis. However, we do not recommend widespread use of our protocol of enhanced AD care in the PACI study for food allergy prevention in a real-world setting. In a real-world setting, we adopted a personalized staged approach to AD treatment according to each infant’s morphology, distribution, severity, and treatment response.
Not recommended maternal allergenic food eliminations
Although the Cochrane Review did not directly evaluate food allergies as an outcome of maternal allergenic food elimination, it found that maternal food elimination during pregnancy and lactation did not prevent food allergen sensitization or the development of eczema and asthma [55]. According to the European Academy of Allergy and Clinical Immunology guidelines, maternal allergenic food elimination during pregnancy and lactation is not recommended for preventing the onset of food allergies [56].
Breastfeeding and infant formula
Although breastfeeding has many benefits for both infants and mothers, Lodge et al. [57] found no association between breastfeeding duration and food allergies in a systematic review and meta-analysis of observational studies. An RCT by Sakihara et al. [58] showed that consuming at least 10 mL of regular milk per day, in addition to breast milk, at ages 1–2 months reduced the onset of milk allergy.In a systematic review and meta-analysis, Boyle et al. [59] showed that partially or fully hydrolyzed milk was not associated with the development of food allergies compared with regular milk. An RCT by Urashima et al. [60] showed that the addition of amino acid formula for 3 days after birth to otherwise exclusive breastfeeding was associated with a lower risk of cow’s milk allergy at 1 year of age than the addition of 5 mL or more of regular milk per day for 3 days after birth. The World Health Organization (WHO) recommends exclusive breastfeeding for at least the first 6 months of life for all children worldwide including those in developed and developing countries. However, along with evidence from clinical trials, this suggests that avoidance of cow’s milk formula during the first few days after birth and the delivery of a small amount of cow’s milk formula beginning at 1 month of age might prevent milk allergy.
Weaning food introduction in infants
There is no evidence suggesting that delaying weaning foods prevents the onset of food allergies. Nwaru et al. [61] showed that delaying the introduction of solid foods was associated with food allergen sensitization. In a systematic review, Burgess et al. [62] found that starting solid foods other than milk before versus after 4 months of age was not associated with the development of food allergies. Currently, local guidelines recommend different timings for the introduction of solid foods to infants [63]. The Asia Pacific Academy of Pediatric Allergy, Respirology, and Immunology recommends solid food introduction at 6 months of age [64], while the Canadian guidelines recommend solid food introduction at approximately 6 months. In contrast, the United Kingdom guidelines recommend solid food introduction at 4 months of age [63]. As we mentioned before, exclusive breastfeeding is suggested by the WHO, and the recommendation of a duration of 4–6 months for allergy prevention varies among guidelines.
Oral ‘single’ allergen introduction—hen’s egg in infants
The Learning Early About Peanut allergy trial was the first to report that early peanut intake in high-risk infants prevented the subsequent onset of a peanut allergy [65]. Based on this evidence, various food allergy guidelines recommend early single allergen food intake to prevent food allergies [56,66-68]. Similarly, regarding the early introduction of hen’s eggs, we reported that a tiny portion of cooked hen’s eggs fedto infantswithADat 5–6months of age significantly reduced the incidence of hen’s egg allergy versus avoiding hen’s eggs until 12 months of age (Prevention of Egg Allergy in High-risk Infants with Eczema study) [69]. Our trial documented no adverse events related to the interventions. We believe that the successful prevention point was that eczema among infants could become controlled through proactive therapy along with a small dose of hen’s egg. Four RCTs [70-73] evaluated the preventive effect of early ingestion of hen’s eggs as a single allergen using a large portion of raw eggs; reportedly, some children developed anaphylaxis. A small dose of allergenic food has sufficient preventive effects. A recent observational study demonstrated that the consumption of hen’s eggs ≥2 times per week (not every day) in late infancy reduced the child’s risk of consequent egg allergy [74]. We believe that food allergies can be prevented even if infants with well-controlled eczema safely eat only small amounts of allergenic foods.
Oral ‘multiple’ food allergen introductions in infants
In the Enquiring About Tolerance study [75] of exclusively breastfed infants who were not considered at high risk of developing food allergies, the early induction group, which started on mixed allergenic foods (at least 2 g weekly of food proteins each of peanuts, cooked eggs, cow’s milk, sesame, whitefish, and wheat) at 3–5 months of age did not demonstrate a reduced onset of food allergies compared to the standard group, which started the foods after 6 months of age. Because the dropout rate from the protocol was high, it was unfeasible to use a large proportion of allergenic foods. Nishimura et al. [76] conducted an RCT of high-risk infants aged 3–4 months with AD. In this trial, the early introduction group received allergenic food powder (containing tiny doses of hen’s eggs, milk, wheat, soybean, buckwheat, and peanut powder) from the age of 3–4 months, whereas the control group received placebo powders. The powder dosage was gradually increased (2.5, 7.5, and 20 mg of each allergenic food protein) throughout the 12-week trial among infants with well-controlled eczema. The early multiple allergenic food introduction group showed a significantly reduced onset of food allergies. Similar to the results of our previous trial of egg allergy prevention [18], a small dose was sufficient to induce immune tolerance.
Quake et al. [77] conducted a pilot study of healthy infants without current food allergies to preliminarily evaluate the safety of 10 mixed food allergen proteins. They tested the early introduction of single foods (milk, eggs, or peanuts), two foods (milk/egg, egg/peanut, or milk/peanut), and multiple foods (milk/egg/peanut/cashew/almond/shrimp/walnut/wheat/salmon/hazelnut at low, medium, or high doses). Adherence was as high as 95%, with no cases of anaphylaxis. Safety and feasibility were satisfactory. These results indicate that even low doses of the mixed powder featured a sufficient preventive effect. Furthermore, mixed-powder interventions outperformed single and 2 allergenic food interventions.
Multiple allergenic food products recently became commercially available as early allergen introduction foods. Many of these agents are marketed as aids for the prevention of food allergies. However, there are concerns regarding safety among infants in the absence of a medical assessment; in fact, there have been several reports of allergic reactions to commercial food products [78].
Conclusion
Although several studies have investigated the preventive efficacy of oral supplements, such as vitamin D, prebiotics, and probiotics, several guidelines do not recommend supplements, prebiotics, and probiotics for food allergy prevention owing to the low certainty of evidence [56,79,80]. We have been conducting an online parental preparation class to implement the latest findings of various studies [9]. We believe that the major invaluable interventions for food allergy prevention are eczema management and the early introduction of small doses of allergenic foods. In daily practice, we can almost completely prevent IgE-mediated food allergies (to hen’s eggs, milk, wheat, and soy)in infants with early-onset eczema [81]. The new nutrition science has a holistic paradigm shift because it is informed by integrating social (including cultural, economic, and political) dimensions with “classical” biological (biochemical, physiological, and medical) dimensions [81]. We should promote infant nutrition should be promoted not only from a classical biological perspective but also from cultural, economic, and political perspectives.The flow of the food allergy prevention strategy (Fig. 6) is recommended in daily practice based on the dual allergen exposure hypothesis—both skin and oral interventions for food allergy prevention. To overcome the food allergy epidemic, pediatricians must become specialists in the treatment of AD with holistic approach [82].

Preventing food allergies in children. The timing of interventions may be modified for each infant. It is difficult to specify the target populations for individual interventions. In a real-world setting, to prevent food allergies in infants, we recommend a personalized approach involving both skin intervention (eczema treatment to achieve early remission and well-controlled skin with no eczema to prevent percutaneous immunoglobulin E sensitization) and oral intervention (early allergenic food introduction) to avoid adverse events based on the dual allergen exposure hypothesis.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This review was partially supported by the 2020 Donation-313 Takahiko Miwa (to Kiwako Yamamoto-Hanada, National Center for Child Health and Development, Tokyo, Japan).
Author contribution
KYH wrote the first draft of the manuscript. KYH and YO approved the final version of the manuscript.
Acknowledgements
This review was previously presented at the 2022 Annual Autumn Congress of Korean Academy of Pediatric Allergy and Respiratory Disease in October 2022.