Clinical practice guidelines for attention-deficit/hyperactivity disorder: recent updates
Article information
Abstract
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common neurodevelopmental disorders found in children and adolescents. The American Academy of Pediatrics (AAP) first published a clinical practice guideline on ADHD in 2000, which was revised in 2011 and republished together with an accompanying process-of-care algorithm. More recently, the 2019 clinical practice guideline revision was published. Since the 2011 guideline, the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), was released. In addition, the Society of Developmental and Behavioral Pediatrics (SDBP) recently released another clinical practice guideline for complex ADHD. Although there are nonessential changes reflected in these updates, a number of changes have still been made; for example, the DSM-5 criteria lowered the diagnostic threshold for ADHD in older teens and adults. Additionally, the criteria were revised to facilitate application to older teens and adults, and a comorbid diagnosis with autism spectrum disorder is now allowed. Meanwhile, the 2019 AAP guideline added the recommendation related to comorbid conditions with ADHD. Lastly, SDBP developed a complex ADHD guideline, covering areas such as comorbid conditions, moderate-to-severe impairment, treatment failure, and diagnostic uncertainty. In addition, other national ADHD guidelines have been published, as have European guidelines for managing ADHD during the coronavirus disease 2019 pandemic. To facilitate ADHD management in a primary care, it is important to provide and review clinical guidelines and recent updates. In this article, we will review and summarize the recent clinical guidelines and their updates.
Key message
· Primary pediatricians should play a key role in the diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD).
· The Diagnostic and Statistical Manual of Mental Disorders, fifth edition, has lowered the diagnostic threshold for older teens and adults and a comorbid diagnosis with autism is now allowed.
· The American Academy of Pediatrics had added recommendation-related comorbid conditions in its guideline and the Society of Developmental and Behavioral Pediatrics recently developed a complex ADHD guideline.
· The European ADHD Guideline Group recently developed a guideline for managing ADHD during the coronavirus disease 2019 pandemic.
Graphical abstract. Summary of the recent ADHD clinical guidelines and their updates. ADHD, attention-deficit/hyperactivity disorder; AAP, the American Academy of Pediatrics; NICE, National Institute of Health and Care Excellence; SDBP, Society for Developmental and Behavioral Pediatrics; EAGG, European ADHD Guideline Group; COVID-19, coronavirus disease 2019.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common neurodevelopmental disorders found in children and adolescents, with a prevalence estimated to be 7% to 10% and increasing gradually [1-3]. Thus, the pediatrician and primary care clinician (PCC) have an important role in ADHD care. The American Academy of Pediatrics (AAP) first published a clinical practice guideline on ADHD in 2000, which was revised in 2011 and republished with an accompanying process-of-care algorithm (PoCA). More recently, the 2019 clinical practice guideline revision was published [4].
Since the 2011 AAP guideline was introduced, the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), was released and new ADHD studies have been published. There have not been considerable changes and the 2019 guideline update includes only incremental revisions. However, it does include the addition of a key action statement (KAS) related to comorbid conditions and a supplemental article on systemic barriers related to ADHD care. In addition, the Society of Developmental and Behavioral Pediatrics (SDBP) recently released another clinical practice guideline for managing complex ADHD, including comorbid conditions [5].
To facilitate ADHD management in primary care, it is important to provide and continue to review clinical guidelines and recent updates.In this article, we will review and summarize the recent clinical guidelines and updates.
Updates to the DSM-5 concerning ADHD diagnostic criteria
As noted, there have not been essential changes made in the DSM-5 concerning ADHD. However, some alterations exist as indicated below.
(1) ADHD has been placed in "neurodevelopmental disorders," a new category created in the DSM-5.
(2) Examples were added to the criteria to facilitate application throughout the patient’s life.
(3) Age at the onset of symptoms was broadened from younger than 7 years to younger than 12 years.
(4) A symptom threshold change was made for older teens and adults (>17 years),where a cutoff of 5 core symptoms, rather than 6, is required both for inattention and/or hyperactivity/impulsivity.
(5) A comorbid diagnosis with autism spectrum disorder is now allowed.
(6) The presence of the symptom is emphasized instead of impairment; evidence of symptoms, instead of impairment, is required in 2 or more settings.
(7) Functional impairment is that which reduces the "quality of social, academic, or occupational function." In the previous version (Diagnostic and Statistical Manual of Mental Disorders, fourth edition [DSM-4]), confirmation of functional impairment required it to be "clinically significant."
(8) Three types of ADHD are now referred to and specified as "presentations."
(9) The severity of the disorder as either mild, moderate, or severe can be specified.
The DSM-5 may have enhanced the chance of diagnosis of patients (especially older teens and adults) with ADHD who have previously failed to receive a diagnosis and treatment. However, the lack of clinical field trials remains a concern [6]. ADHD symptoms with comorbid autism have been reported in 30% to 50% of patients; thus, autism was removed as an exclusion criterion [7]. Additionally, the advantage of changing "subtypes" to "presentations" highlights that the presentation of a patient with ADHD may change at different points during the lifespan. Table 1 provides a brief overview of the major changes from DSM-4 to DSM-5.
Updates to the 2019 AAP guideline
The 2019 AAP guideline introduced 7 KASs for the evaluation, diagnosis, and treatment of ADHD in children and adolescents [4]. In this edition, the first 6 KASs remain essentially unchanged from the previous guideline; however, KAS 7, relating to comorbid conditions, was newly added in the 2019 guideline. The accompanying PoCA was also updated (Fig. 1) and a supplemental article has been added on systemic barriers related to ADHD care. Updates made in the 2019 AAP guideline are detailed in the following paragraphs.

Flowchart of ADHD diagnosis. ADHD, attention-deficit hyperactivity disorder; KAS, key action statement; PCC, primary care physician; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, fifth edition.
1. Evaluation and Diagnosis for ADHD
The first 3 KASs are related to the evaluation and diagnosis of ADHD. As noted, ADHD is the most common neurodevelopmental disorder. Pediatricians and other PCCs should play a key role in the evaluation and diagnosis of ADHD [8,9]. The process requires establishment of a sustained relationship with child and family. The child with a history of ADHD risks (e.g., extremely low birth weight, family history of ADHD) should be monitored in a primary care setting. Many parents bring their child to the primary care clinic with concerns about ADHD. Concerns may also be identified during routine screening at preventive visits. Additionally, parents may visit the clinic at the recommendation of a teacher or other caregiver. In these situations, the pediatrician or PCC should initiate an evaluation for ADHD.
A diagnosis of ADHD should be made based on DSM-5 criteria, including documentation of symptoms and impairment in more than one major setting. The clinician should identify the chief concerns and history of symptoms, and the family, medical, and psychosocial history should be reviewed. Relevant information may also be obtained from the diagnostic interview, school and other reports, and academic records. DSM-5–based rating scales could be helpful for collecting information. There are minimal changes in ADHD behaviors from the prior DSM publication (DSM-4); thus, other DSM-based rating scales can be used.
In the evaluation for ADHD, comorbid conditions should be screened for. A wide range of emotional or behavioral conditions, developmental conditions, and physical conditions can be comorbid with ADHD [10-17]. These conditions may affect the treatment for ADHD and also mimic ADHD symptoms. Detailed history-taking and a review of systems are required, which might reveal other disorders or problems (e.g., sleep problems, developmental delay, or trauma history). Hearing and visual problems also need to be screened for, and internalized symptoms, such as anxiety and depression, may be noticed during the interview with the child or adolescent. Information from the child’s school might reveal comorbid or mimicking conditions, such as depression and anxiety, disruptive behavior disorders, or learning disabilities. Recording clinical observations and conducting a physical examination are important as the patient may present with language disorders, developmental disorders, or other physical conditions such as genetic syndromes.
2. Treatment for ADHD
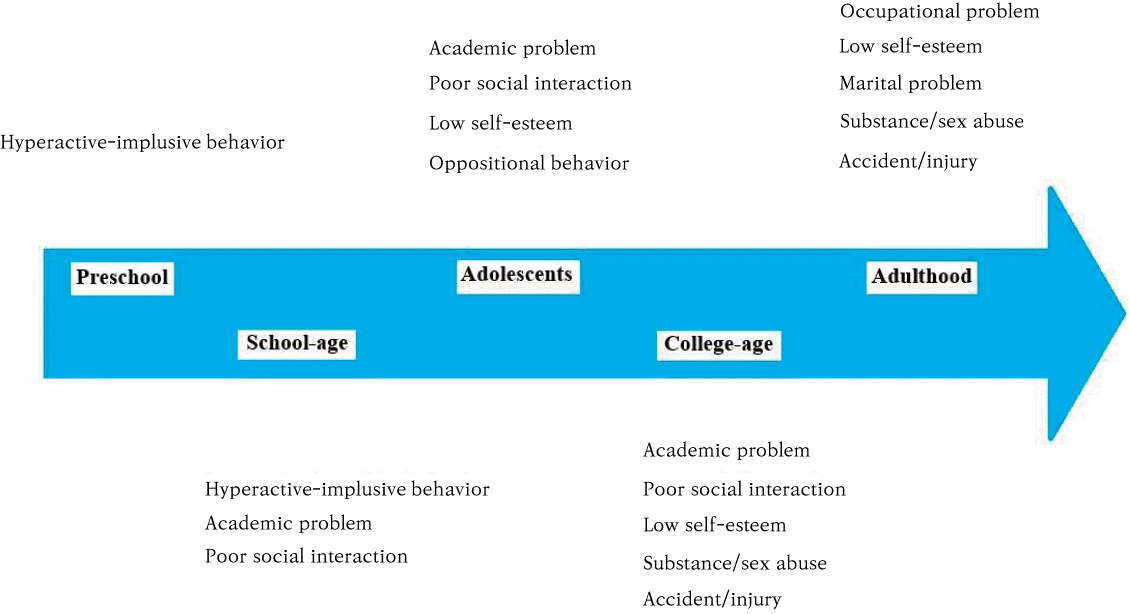
The fourth, fifth, and sixth KASs are related to the treatment of ADHD. ADHD is a chronic condition, such as asthma, and affected individuals have special health care needs (Fig. 2). The AAP suggested the principles of the chronic care model and the medical home for such chronic conditions (Fig.3) [18-20]. ADHD should be managed according to the same principles. This model may be beneficial, especially for parents with ADHD or those with a low socioeconomic status. Further, it may be helpful in maintaining ADHD treatment. It is important to establish a team approach involving the child and their family, school personnel, and mental health clinicians.

Flowchart of ADHD treatment. ADHD, attention-deficit hyperactivity disorder; KAS, key action statement; PCC, primary care physician; PTBM, parent training in behavior management; IEP, Individualized Educational Program; FDA, U.S. Food and Drug Administration.
For children aged 4 to 5 years, parent training in behavior management (PTBM) and/or behavioral classroom interventions is recommended as a first-line ADHD treatment [21,22]. Weekly courses are designed to help parents learn age-appropriate developmental expectations, behaviors that strengthen the parent-child relationship, and specific management skills for problem behaviors. One example of evidence-based PTBM is parent-child interaction therapy, a dyadic therapy for parents and children. PTBM is a group or individual program on a weekly basis. The programs offer specific techniques in behavior management. PTBM is applicable even without a definite ADHD diagnosis and can help parents to learn age-appropriate development. Subsequently, it may inform the diagnostic evaluation for ADHD. Thus, PTBM is also recommended if an ADHD diagnosis is doubtful. Moreover, training intervention has recently emerged as an ADHD treatment modality, targeting specific deficient behaviors and skills. In this context, extensive training, practice, and coaching are provided. Overall, a combination of PTBM and training interventions may be most effective. PTBM and training interventions can also be applied to address behavior problems that do not fully meet the DSM-5 criteria.
Methylphenidate can be considered for children aged 4 to 5 years in the following cases: (1) moderate-to-severe ADHD that did not respond to behavioral interventions and (2) in areas in which evidence-based behavioral treatments are not available. However, the clinician needs to assess the benefits and harms of starting such medication. Only methylphenidate has sufficient evidence regarding its use in this age group [2,22]. However, it should also be noted that the evidence does not yet meet the approval level for the U.S. Food and Drug Administration (FDA). Other stimulant or nonstimulant medications have also not been adequately studied [23]; thus, they are not recommended for use in preschool-aged children.
For children aged six to 11 years, the guideline suggests the use of FDA-approved medications for ADHD, together with PTBMand/or behavioral classroom intervention (preferably both). An FDA-approved stimulant (methylphenidate or amphetamine) is the first choice in medication treatment [24]. These 2 stimulant medications have similar benefits and adverse effects; however, only methylphenidate is currently available in Korea. Meanwhile, FDA-approved nonstimulant medications include norepinephrine reuptake inhibitors (atomoxetine) and selective α-2 adrenergic agonists (e.g., guanfacine or clonidine) [25-28]. Nonstimulant medications have also demonstrated sufficient, albeit less robust, efficacy (i.e., the ratio of stimulants:nonstimulants effect size to control is 1.0:0.7) [29]. Behavioral treatment in combination with medication has positive effects [30,31]. This combined treatment approach is beneficial, especially for children with comorbid anxiety or living in low socioeconomic environments. The effects of behavioral therapies tend to persist, but the effects of medication cease when administration stops. Moreover, behavioral therapy allows for a lower medication dosage to be prescribed [32]. In school-aged children, school programming (educational intervention and/or accommodation) and supports are necessary parts of the treatment plan [20]. The clinician needs to collaborate with the child’s school to enhance supports and services.
For adolescents aged 12 to 18 years, FDA-approved medications, behavioral/training interventions, and educational interventions/supports are also recommended. In adolescents, a special concern should be taken to provide long-term medication coverage. Long-acting or supplemental short-acting medications can be helpful. Long-acting or additional short-acting medications can be helpful. The clinician needs to communicate bidirectionally with the adolescent patient. Transition to adult care should also be prepared.
Lastly, in its ADHD treatment guidelines, the AAP recommends that the clinician should titrate doses of ADHD medications to reach their maximum benefit with tolerable side effects. The Multimodal Treatment of Attention-deficit Hyperactivity Disorder (MTA) study demonstrated that, when the full range of methylphenidate was administered, ADHD symptoms improved in more than 70% of study participants [31]. Stimulants can be effectively titrated on a weekly basis; in urgent cases, this may be completed within a three-day period. Follow-up visits are recommended monthly until the optimal response plan is developed, then every 3 months thereafter within the first year. Subsequent visits are suggested at least twice a year. After several years, the medication can be tapered. A patient who does not respond to stimulants may still respond to nonstimulant medications. Atomoxetine elicits a maximal response approximately 4 to 6 weeks after initiation, while guanfacine and clonidine demonstrate maximal effects about 2 to 4 weeks after; however, only guanfacine and clonidine have been approved as adjunctive therapy to stimulants by the FDA [33]. Other combinations may be used on an off-label basis. The frequent adverse effects associated with stimulants include headache, stomachache, weight loss, and sleep disturbance. Uncommon and serious side effects of stimulants at high doses include hallucinations and other psychotic symptoms. The MTA study showed that height growth diminished in the range of 1 to 2 cm and was not compensated for after [31]. Increases in heart rate (HR) and blood pressure (BP) were clinically insignificant and mild [34]. The risk of sudden cardiac death was not increased [35-39]. Nevertheless, the clinician should monitor both the patient's HR and BP along with their height and weight. Before initiating therapy with stimulant medications, it is important to obtain the patient's history of cardiac symptoms and a family history of sudden cardiac death and cardiovascular symptoms. If these risk factors are present, the clinician should consider further evaluation. The frequent adverse effects of atomoxetine include somnolence, abdominal pain, and weight loss [40-42]. Height growth delays may existin the first one to 2 years but are compensated for after [43]. Increased suicidal thoughts have infrequently been found, and this black box warning should be noted. For guanfacine and clonidine, the adverse effects include somnolence, dry mouth, abdominal pain, dizziness, headache, bradycardia, and hypotension [8,44,45]. Because of rebound hypertension,these medications should be tapered off [46].
3. Comorbid Conditions and ADHD
KAS 7 is related to comorbid conditions in patients with ADHD and was newly added in the 2019 ADHD guideline. The AAP highlights that the primary pediatrician needs to take responsibility for mild-to-moderate ADHD, anxiety, depression, and substance use. The accompanying PoCA suggests several validated scales for comorbid conditions. ADHD rating scales also include items for screening for comorbidities. AAP mental health initiatives provide the algorithm for broader mental health. KAS 7 is integrated with this algorithm. The primary pediatrician may not be trained or experienced enough to manage ADHD with comorbid conditions. Patients with complex ADHD with severe comorbidities should be referred to a specialist. Those with urgent conditions, such as suicidal attempt, self-injury, and temper outbursts, need to be transferred immediately. The primary pediatrician should still be prepared and participate as a team member in these situations.
2020 SDBP Complex ADHD Guideline
In 2020, the SDBP first published a clinical practice guideline on a complex ADHD [5,47]. In this guideline, a complex ADHD was defined based on age (<4 years or presentation at ≥12 years), coexisting conditions, moderate-to-severe functional impairment, diagnostic uncertainty, or inadequate response to treatment. The accompanying PoCA was also developed. The algorithms included complex ADHD with comorbid conditions (e.g., substance use, anxiety, depression, disruptive behavior, tics, and autism). This guideline focused on subspecialty-level care, but it can also aid primary pediatricians in a complex ADHD care.
Other national ADHD guidelines
Several national guidelines from Europe and North America, have been published for ADHD in children and adolescents. Although based on different healthcare systems and cultures, the core recommendations are similar throughout the current clinical guidelines [48]. Apart from the Canadian guidelines, which did not include a systematic review of the evidence, European guidelines (United Kingdom [UK], German, Dutch, and Spanish) were developed by combining evidence summaries (systematic reviews, meta-analyses, and assessment of quality of evidence). In addition, the UK National Institute of Health and Care Excellence (NICE)49) and German guidelines provided expert opinions, the Dutch guidelines included individual studies, and the Spanish guidelines referenced recommendations from other guidelines, including NICE and AAP. The NICE and Dutch guidelines included health economic evidence when developing recommendations.
While North American guidelines include primary care physicians, European guidelines refer to the specialized training of pediatricians and psychiatrists for ADHD. The UK NICE guideline also allows shared care plans between specialists and primary care physicians. All guidelines provide recommendations by age group (e.g., preschool age, older children, and adolescents). The UK, German, and Spanish guidelines classify participants as school-aged children (under 18 years) and adults (age 18 years and over). The UK NICE guideline defines young children as those younger than 5 years. Most guidelines suggest that stimulants are first-line drugs, but the Spanish guideline only indicates that approved drugs can be used without a recommended priority. In contrast to the other guidelines, the NICE 2018 guideline no longer recommends parent training as first-line treatment in school-aged children because of the lower effect sizes and poorer quality of evidence compared to that for medication. However, ADHD-focused parent training remains the first-line intervention for preschool-aged children.
ADHD management during the COVID-19 pandemic (Table 2) [50-58]
The European ADHD Guidelines Group (EAGG) recently developed a guideline for managing ADHD during the coronavirus disease 2019 (COVID-19) pandemic [59]. Children and adolescents with ADHD are particularly suffering from the pandemic and social distancing and might have increased behavioral problems. All related services must be continued via telephone or online video. Schools and teachers need to monitor all students, but give priority to students with ADHD, especially adolescents, because of their disabilities and increased level of risk. Behavioral parenting strategies are recommended at home because they have beneficial effects in child behavior and reducing disruptive behavior. Individuals using cognitive and behavior training should be encouraged to continue practicing at home despite the new challenges. The EAGG recommends flexible application of restrictions on access to ADHD medications during the COVID-19 outbreak to ensure that patients receive their medications in a timely manner. Parents of children with ADHD and adolescents or adults with ADHD should not change their medication doses to manage stress related to confinement unless prescribed by a doctor. Routine face-to-face cardiovascular monitoring in patients with ADHD without risk factors can be delayed until in-person visits are resumed. Currently,the risks of cardiovascular monitoring outweigh the benefits in this patient group. If possible, it is recommended to use home BP and HR monitors.
Conclusions
The pediatrician and primary physician have important roles in ADHD care. ADHD is the most common neurodevelopmental disorder. As primary pediatricians might have close relationships with both the child and their family, and there are many opportunities to identify ADHD in a primary care. The pediatrician and primary physician should adopt a key role in the evaluation, diagnosis, and treatment of ADHD. A team approach involving the child and the family, school personnel, and mental health clinicians needs to be established. This article will help facilitate ADHD care by the primary pediatrician and provide a training program, resources, and an ADHD care model for use in a primary care.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; Ministry of Science and ICT) (no. 2020R1G1A1099968).