Heart failure in children and adolescents: an update on diagnostic approaches and management
Article information
Abstract
Cardiac failure is a clinical syndrome that may develop in children owing to cardiac dysfunction or underlying structural heart diseases. Considering the differences in diagnostic and therapeutic approaches for pediatric heart failure (PHF) and adult heart failure, we have reviewed the current literature on PHF. Relevant studies were extracted from MEDLINE/PubMed, Google Scholar, and Clinical Trial Registries using the terms “pediatric heart failure” or “heart failure in children” and “management” or “decongestive therapy.” Recent advances in diagnostic approaches, such as cardiac magnetic resonance, speckle-tracking echocardiography, tissue Doppler imaging, and molecular diagnostic techniques, have increased our under -standing of PHF. It is imperative that clinicians evaluate the interrelated factors responsible for the develop ment of PHF, including myocardial function, pulmonary and systemic blood flow, heart rhythm, valve function, and nutritional status. Although recent advances have demon strated the efficacy of many new drugs in adult heart failure trials, it cannot be concluded that these drugs will show similar efficacy in children, considering the heterogeneous nature of the underlying mechanisms and variable pharmacody-namics and pharmacokinetics. Therefore, the underlying pathophysiology of PHF and the mechanisms of action of different drugs should be considered when selecting appropriate therapies. Further trials are needed to establi sh the efficacy and safety of these drugs, and a combined mul-ti disciplinary strategy will help enhance PHF outcomes.
Key message
· Pediatric heart failure (PHF) is a clinical syndrome featuring various symptoms (shortness of breath, ankle swelling, fati gue) and signs (pulmonary crackles, peripheral edema).
· Congenital heart diseases are the most common underlying etiology of PHF, whereas myocarditis and primary cardio-myopathies are common in children without structural ab-normalities.
· PHF pathophysiology is complex and multifactorial and varies by etiology and age.
· PHF management includes decongestive therapy, treatment of underlying causes, preventing progression, and managing pulmonary or systemic obstructions.
· Drugs should be chosen based on pharmacodynamics, clinical manifestations, hemodynamic state, and renal function.
Introduction
Low cardiac output was identified in the 1950s as a clinical syndrome that leads to heart failure (HF) [1]. In recent years, researchers have shown more interest in identifying the role of neurohormonal and molecular mechanisms affecting cardiac function in failing hearts [2]. Pediatric HF (PHF) may develop secondary to structural problems, such as congenital heart disease (CHD) and cardiomyopathies, or cardiac dysfunction due to infectious and inflammatory diseases,metabolic syndromes,malnutrition,malignancies, and renal failure [3,4]. Despite being well-recognized, data on the prevalence and incidence of PHF are limited. Although PHF has a low incidence (0.9–7.4 cases per 100,000 children), morbidity and mortality remain high with a 7%–26% in-hospital mortality rate [5,6]. In patients younger than 18 years, infants comprise most PHF admissions (64%) [4]. In a systematic review of 83 studies, Shaddy RE et al. [6] found a wide variation in the reported incidence of PHF, ranging from 0.87 per 100,000 in the UK and Ireland to as high as 83.3 per 100,000 in Spain.
The development of various noninvasive, minimally invasive, and modern radiological methods and pharmacological advances have aided the better management of PHF. Drugs such as angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARBs), β-blockers, mineralocorticoid receptor antagonists, angiotensin receptor neprilysin inhibitors (ARNI), and sodium-glucose cotransporter-2 (SGLT-2) inhibitors have completely transformed the management of HF. Although the efficacy of these drugs has been well documented in adult HF trials, similar pediatric trials have yet to conclude that these drugs show similar efficacy in children due to the heterogeneous mechanisms of PHF and its variable pharmacodynamics and pharmacokinetics. Therefore, we aimed to provide an up-to-date review of the current developments in the diagnosis and management of PHF.
Review
We retrieved relevant information from studies focusing on PHF and its treatment by performing extensive literature searches of the MEDLINE, PubMed, and Google Scholar databases. The following search terms were used: “pediatric heart failure” or “heart failure in children” and “management” or “decongestive therapy.” The reference lists of the collected studies were manually checked to retrieve any additional relevant studies.
Definitions
1. Heart failure
According to European Society of Cardiology recommendations in 2021, HF is not a discrete pathological diagnosis but rather a clinical syndrome characterized by a collection of symptoms (e.g., shortness of breath, ankle swelling, fatigue) and occasional comorbid signs (e.g., elevated jugular venous pressure, pulmonary crackles, peripheral edema) [7].
2. Acute HF
HF is labeled “acute” when signs and symptoms of malperfusion and congestion, such as tachycardia, tachypnea, respiratory distress, and hypotension, appear suddenly (within minutes to hours), usually because of anatomical or functional changes in the heart [8].
3. Chronic HF
Chronic HF (CHF) is a progressive condition that may have both cardiac and noncardiac causes. Respiratory distress, pedal edema, exercise intolerance, and growth failure are symptoms indicative of underlying neurohormonal, circulatory, and molecular abnormalities [4].
CHF is classified as HF with preserved ejection fraction (HFpEF) or HF with reduced ejection fraction (HFrEF). HFrEF, characterized by symptomatic HF with a dilated left ventricle (LV) and an LV ejection fraction (LVEF) <50%, is most often caused by LV systolic dysfunction. Symptomatic HFpEF, which features normal or near-normal LV systolic function, is usually caused by severe LV diastolic dysfunction and is also known as diastolic HF [2,4].
Etiology
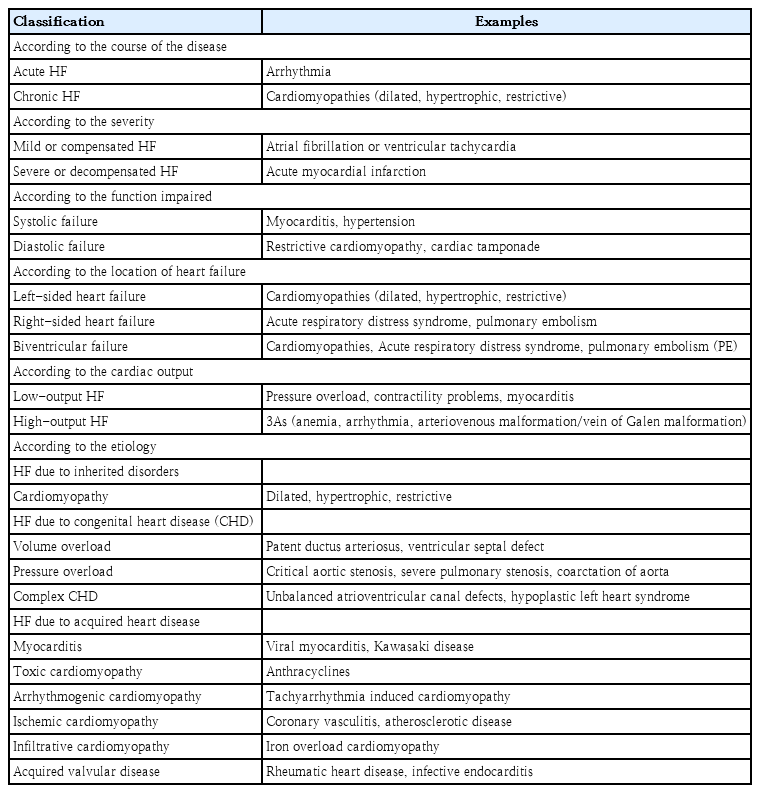
The etiology of HF varies widely between adults and children. Ischemic heart disease is a common underlying cause of HF in adults, but it rarely causes HF in children.In contrast, structural heart diseases, especially CHD, are the most common underlying etiology of PHF [8], while children with structurally normal hearts are most often affected by myocarditis and primary cardiomyopathies [3,4]. The most common morphological phenotypes of cardiomyopathy are dilated (60%) and hypertrophic (25%). In developed countries, the primary causes of PHF include CHD and cardiomyopathy, whereas rheumatic heart disease and infective endocarditis remain common causes in developing countries. Table 1 summarizes the various etiologies of PHF. Common causes of HF at birth include fetal cardiomyopathies and extracardiac disorders (e.g., sepsis, hypoglycemia, hypocalcemia), whereas ductus-dependent lesions, such as coarctation of the aorta and hypoplastic left heart syndrome, lead to HF in the first week of life. In the first month of life, left-to-right shunts such as ventricular septal defects, patent ductus arteriosus, and aortopulmonary windows are the main causes of PHF [3,4].
Pathophysiology
The pathophysiology of HF in children is complex and multifactorial and varies according to the underlying etiology and the child’s age. In cases of CHD, HF occurs because of alterations in blood flow and hemodynamic overload, leading to ventricular remodeling and dysfunction. Chronic pressure overload results in myocardial hypertrophy, which increases myocardial demand and oxygen consumption. Increased cardiac workload also leads to progressive ventricular dilatation, worsening heart function, and eventual HF. Cardiomyopathies are characterized by myocyte hypertrophy, apoptosis, and fibrosis, resulting in impaired myocardial contractility and relaxation, which manifests as HF. Acquired heart diseases, such as Kawasaki disease, myocarditis, endocarditis, and rheumatic fever, result in HF through inflammation and myocardial injury, leading to valvular dysfunction and decreased myocardial contractility and cardiac output. In valvular heart disease, regurgitation and stenosis cause volume and pressure overload, respectively, resulting in ventricular remodeling and dysfunction [9,10].
Regardless of the etiology, HF symptoms result from inadequate cardiac output. The body attempts to maintain cardiac output by activating compensatory mechanisms involving the renin-angiotensin-aldosterone system (RAAS), sympathetic nervous system (SNS), and natriuretic peptides. SNS releases catecholamines and increases heart rate, systemic vascular resistance, and myocardial contractility, whereas RAAS activation releases angiotensin II and aldosterone, causing sodium and water retention and leading to increased preload and ventricular dilation. Atrial natriuretic peptide and B-type natriuretic peptide (BNP) promote natriuresis and diuresis and inhibit renin secretion and aldosterone production. These molecules also inhibit cardiac remodeling, apoptosis, and fibrosis. Over time, these compensatory mechanisms become maladaptive, leading to progressive ventricular dysfunction and worsening of HF through adverse cardiac remodeling. The release of proinflammatory cytokines and chemokines also contributes to myocardial damage and inflammation [9,10].
Clinical presentations
The clinical presentation of HF in children can vary widely by age, underlying etiology, and disease severity. Patients with PHF commonly present with respiratory symptoms such as dyspnea, tachypnea, and respiratory distress, which may be exacerbated by physical activity or feeding. Circulatory symptoms include peripheral edema, hepatomegaly, and pleural effusion. Growth retardation and failure to thrive can occur in infants with HF due to increased metabolic demands, whereas older children may experience palpitations, chest pain, and syncope due to decreased cardiac output and arrhythmias. These children may exhibit signs of cerebral hypoperfusion such as irritability, altered mental status, and lethargy. Peripheral cyanosis and pallor have also been observed [3,4,9,10].
Diagnostic methods
Noninvasive clinical examinations are initially performed to diagnose PHF; however, early diagnosis is difficult owing to a lack of sensitivity and specificity. A few commonly used noninvasive methods are listed below.
1. Chest radiography
Chest radiography is recommended for children with suspected HF to determine the size and shape of the heart and identify signs such as pulmonary edema, septal lines (also known as Kerley B lines), and pleural effusions [5].
2. Echocardiography
Owing to its widespread availability in most centers, echocardiography is the most widely used and economically advantageous test for confirming an HF diagnosis.It is used to evaluate myocardial wall thickness, cardiac chamber diameter and volume, ventricular systolic/diastolic function, and pulmonary pressure [2]. It also aids determination of the underlying cause of HF via capturing the anatomy and morphology of the heart, valves, major arteries, and surrounding tissues [2]. M-Mode, 2-dimensional echocardiography (2D-echo), and traditional Doppler imaging are typical echocardiographic procedures.
Several challenges unique to pediatric echocardiography include complicated anatomy and difficulty evaluating cardiac function in ventricles with varying morphologies. More recent advancements in pediatric echocardiography include non-Doppler-based (2D) stress and strain rate imaging, 3-dimensional echocardiography (3D-echo), functional imaging, and imaging of cardiac deformations. High spatial- and temporal-resolution imaging is possible using modern ultrasound technology, especially in pediatric probes, and it provides a useful window into myocardial mechanics and function [11].
In a 2D image, the motion can be analyzed by observing speckles (natural acoustic markers). A change in the geometry of each speckle reflects the motion of the adjacent tissue [12]. Speckle velocity may be computed from the change in location after the frame rate has been established, and this motion pattern of speckles reflects the motion pattern of the cardiac tissue. Consequently, it is feasible to calculate the stress and strain rates by observing these speckles [13]. The connection between the echocardiographic speckle-tracking approach and the well-known optical imaging modality of particle image velocimetry has enabled the creation of a novel technique for observing LV flow patterns. The velocity vector field in LV may be sufficiently reconstructed using this angle-independent contrast echo-based approach [13].
However, echocardiography is less reliable for determining the etiology of HF, particularly in children. Owing to its dynamic nature, strain echocardiography may be beneficial for determining the absence of ischemia in uncertain situations involving hypertrophic cardiomyopathy or mitral regurgitation (MR). Using 3D-echo, the precise pathophysiology of several underlying valvulopathies such as mitral valve prolapse may also be explained. Additionally, 3D-echo and functional echoes may help identify complicated anatomical and functional changes and choose appropriate treatments [14]. Many studies have compared 2D- and 3D-echo with a “reference” standard like cardiac magnetic resonance. A recent meta-analysis of all 3D-echo studies evaluating LV volumes and EF revealed that it often underestimates volumes, but not as much as 2D-echo [14].
3. Troponin
Cardiac troponins (cTn) are the primary biomarkers for detecting ischemia or myocardial infarction as well as the etiology of acute HF. Increased blood troponin I and troponin T (TnT) levels suggest severe cardiac damage, particularly acutely decompensated CHF, as both proteins are present in the contractile apparatus of myocytes. However, cTn levels are higher in healthy neonates and infants than in adolescents, while male individuals usually have higher cTn levels than female ones [15]. In a study by Dionne et al. [16], the optimum troponin cutoff value to differentiate between a cardiac and noncardiac diagnosis of HF was greater in children <3 months of age (0.045 ng/mL) than in older infants (0.005 ng/mL). In a previous study, children with myocarditis had considerably higher TnT levels than those with dilated cardiomyopathy (DCM) or HF caused by massive left-to-right shunts [17]. However, normal troponin levels do not exclude the possibility of myocarditis [18].
4. B-type natriuretic peptide
BNP and N-terminal pro-BNP (NT-proBNP) are produced by the myocardium in response to cardiac strain, and children with CHD, cardiomyopathy, or heart transplant rejection have significantly elevated BNP and NT-proBNP levels [15-17]. Despite a lack of substantial randomized trials to prove its efficacy in normal clinical practice, NT-proBNP is a commonly accepted biomarker for HF diagnosis, treatment monitoring, and risk stratification [18-20]. A recent meta-analysis suggested that BNP testing may be an effective screening tool for PHF [21]. Lin et al. [22] found that a combination of the modified Ross criteria scores (≥4) and NT-proBNP levels (≥598 ng/L) could be used to diagnose PHF with 95% accuracy.
5. Cardiac catheterization
Noninterventional pediatric cardiac catheterization is an important tool for accurately assessing hemodynamic conditions in children with CHD. Although not indicated for the diagnosis of PHF, diagnostic catheterization may provide important information about the anatomy and pathophysiology of certain cases of PHF to better manage them [23]. Additionally, in patients with unexplained pulmonary hypertension, right cardiac catheterization helps confirm the diagnosis, assess whether high vascular resistance is reversible, and rule out other treatable reasons. Although imaging studies such as echocardiography and cardiac magnetic resonance imaging (MRI) are becoming important tools for hemodynamic evaluation, they provide only indirect evidence, whereas catheterization can provide direct evidence of LV diastolic dysfunction [24].
6. Endomyocardial biopsy
Owing to its invaluable information on myocardial histology, immunohistochemistry, and molecular structure, endomyocardial biopsy (EMB) is used to ascertain the origin of various cardiac disorders, improve patient stratification, and guide treatment choices. It is helpful for managing patients with unexplained acute HF, hemodynamic compromise, ventricular arrhythmias/conduction problems with unclear causes, and tracking the heart transplant rejection status. Considering the evolution of cutting-edge imaging technology, itis necessary to reconsider the current role of EMB in the study and treatment of cardiovascular illnesses [25].
Although it is considered the gold standard for the diagnosis of myocarditis, the actual indications for EMB in children remain debatable. Thus, this high-risk invasive procedure should be restricted to patients for whom confirmation of the diagnosis of myocarditis is helpful for optimizing therapeutic approaches [26]. Common complications include pericardial effusion, deep venous thrombosis, and third-degree atrioventricular block [27]. The present guidelines are mainly based on case-control studies and expert opinions owing to the lack of prospective studies evaluating the efficacy of EMB in children [26].
Recent advances in diagnostic techniques
1. Cardiac imaging
Evaluations of CHD as the underlying cause of PHF have shown improved accuracy when a computed tomography (CT) scan is used in addition to catheter angiography and echocardiography. In most cases, the extracardiac vascular system is implicated, making CT a better tool than echocardiography for diagnosing complicated CHD [2]. CT angiography offers a noninvasive alternative to catheter-based angiography for visualizing the vascular architecture [2,3]. However, skepticism has been raised about the safety of CT scans for children; however, modern multidetector and dual-source CT has enhanced temporal resolution, leading to quicker scans and less radiation exposure.
MRI provides a radiation-free, noninvasive option for gaining insight into a patient's internal structure and function. Compared to traditional contrast agents such as gadolinium, ferumoxytol may be a safer and more practical option for patients with renal impairment [28]. Younger children may require sedation, which carries its own risks, as cardiac MRI may take 1–2 hours depending on the anatomy. Cardiac MRI may aid in the diagnosis, preoperative evaluation, risk assessment, and treatment of certain cardiac conditions such as cardiomyopathy and complicated CHD via tissue characterization and evaluation [29].
Multidirectional vascular flow patterns and hemodynamics may be detected using MRI 4-dimensional (4D)-flow evaluation. Jacobs et al. [30] showed that 4D-flow evaluation augmented by gadobenate dimeglumine was more accurate than 2D flow evaluation. Right ventricular volumes in children with right tetralogy of Fallot were measured with great reproducibility and accuracy, with a slight overestimation compared to 2D imaging.
2. Molecular diagnostic techniques
Genetic testing for CHD has progressed from the evaluation of a small number oflocito the examination of the whole genome owing to recent developments in bioinformatics, DNA sequencing technology, and the computational infrastructure required to handle genomic data. Sanger sequencing, fluorescence in situ hybridization (FISH), karyotyping, and multiplex ligation-dependent probe amplification are time-consuming methods for genetic investigations [31]. Whole-exome sequencing, chromosomal microarray analysis, and whole-genome sequencing are examples of high-throughput genomic testing methods. FISH can be used to identify macromolecules with high specificity by using probes with known sequences. Current commercially available FISH probes for CHD mostly target aneuploidy and 22q11 deletions [31].
Management of PHF
The principles of PHF management include decongestive therapy, treating underlying causes, preventing progression, and managing pulmonary or systemic obstructions. Appropriate drugs should be chosen based on their pharmacodynamics and patient characteristics such as clinical manifestations, hemodynamic state, and renal function [4,32-34].
1. Supportive management
Patients with CHF are more likely to have anemia and iron deficiency (ID), although these conditions may coexist. Both anemia and ID are associated with severe symptoms and worse clinical outcomes. Furthermore, it is important to recognize that anemia may be the root cause of HF or a factor associated with its development and worsening of clinical conditions. Although ID may be quickly identified using 2 biomarkers (serum ferritin and transferrin saturation), it remains underdiagnosed in patients with PHF. Owing to the low oral iron absorption in these patients, oral iron supplementation is inadequate for treating ID anemia in children with HF [35].
Furthermore, anemia and poor long-term clinical outcomes in HF patients are related to low erythropoietin levels. Studies have shown an increased functional ability and decreased hospitalization in patients with HF and anemia treated with erythropoietin-stimulating drugs. In a meta-analysis by Montero et al. [35], 24 of 25 investigations showed a lower erythropoietin (Epo) ratio in patients having HF with anemia than in normal reference values or patients having HF without anemia.In addition, circulating Epo levels were higher irrespective of hemoglobin levels in patients with HF than in healthy controls.
Lifestyle factors such as poor food quality, obesity, inactivity, and increased emotional stress levels have been implicated in the changing epidemiology of HF. Children with CHD are strongly encouraged by the European Association for Cardiovascular Prevention and Rehabilitation to adopt light-to-moderate levels of physical activity, including recreational sports and fitness training. Clinical improvement from PHF therapy and management may also be influenced by a patient’s commitment to a healthy lifestyle through practices such as yoga and meditation [36].
Acute decompensated heart failure
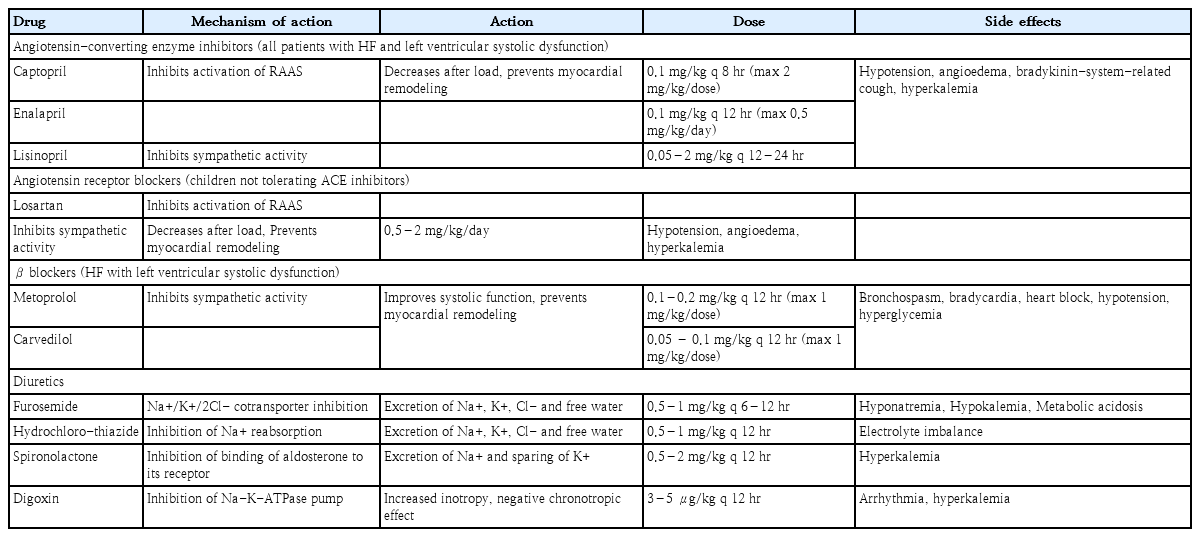
Vasoactive medications, diuretics, and inotropic medicines are the current standards of care for children with acute decompensated HF (ADHF). The details of the drugs used to manage ADHF are summarized in Table 2.
1. Diuretics
Loop diuretics such as furosemide and bumetanide remain the first-line treatments. Recent International Society for Heart and Lung Transplantation (ISHLT) guidelines for managing PHF recommend the initiation of diuretics in patients with fluid retention and ventricular dysfunction, which should be continued until euvolemia is achieved [4,32,33]. Although studies have failed to demonstrate survival benefits in patients with PHF, diuretics are very effective at reducing systemic, pulmonary, and venous congestion and feature a good safety profile. A continuous furosemide infusion may be safer and better in acute HF and postoperative settings.
2. Inotropes and vasoactive drugs
Inotropes are used as rescue therapy in patients with ADHF to prevent end-organ failure by increasing perfusion pressure and allowing diuresis to occur [32,33]. These drugs improve myocardial contractility and increase cardiac output. Digoxin remained the main drug for PHF management for many years before an increased understanding of HF pathophysiology led to a preference for neurohormonal control over inotropic therapy. Currently, other inotropes, such as milrinone, dobutamine, dopamine, and epinephrine, are utilized more frequently, with milrinone and/or dobutamine being the first-line inotropes and epinephrine being used in cases of refractory hypotension and poor end-organ perfusion [32-34].
ISHLT guidelines recommend the use of temporary inotropic support in children presenting with cardiogenic shock, low cardiac output, or poor end-organ perfusion. However, these drugs should only be used as short-term measures of initial stabilization and as a bridge to transplantation or mechanical circulatory support [4]. After stabilization is achieved, oral HF maintenance medication should be initiated to reduce the risk of recurrence and alleviate CHF symptoms [4,32-34].
Although milrinone is widely used to treat ADHF in children, only a few pediatric studies have confirmed its efficacy. Administering milrinone to children with ADHF before CHD surgery improved end-organ perfusion and cardiac output; however, this improvement was short lived in the PRIMACORP (Prophylactic Intravenous use of Milrinone After Cardiac Operation in Pediatrics) study [37]. Extended-release milrinone improved quality of life and functional ability in phase I/II clinical study of adult patients with advanced HF and was well tolerated at 30 days [38].
3. Vasodilators
Vasodilators, such as nitroprusside or nitroglycerin, are indicated in selected cases of ADHF that present with significant cardiac volume overload (e.g., valvular regurgitation) in the absence of hypotension. These drugs significantly improve stroke volume and cardiac output without increasing the myocardial oxygen demand. Nitroglycerin is a prodrug, and nitroprusside exerts a greater effect on peripheral arteriolar dilatation than nitroglycerin [32,33].
4. Calcium sensitizer (levosimendan)
Intravenous levosimendan, a vasoactive drug, improves cardiac contractility and decreases afterload. The activation of sarcolemmal potassium channels in peripheral vascular smooth muscles causes vasodilation and decreases afterload, whereas sensitization of TnC to calcium mediates its inotropic action. Additionally, it protects the heart by activating potassium channels in the cardiomyocyte mitochondria [39].
Small randomized controlled trials (RCTs) have demonstrated improved hemodynamic conditions in pediatric patients treated with levosimendan undergoing cardiac surgery. However, the preventive use of levosimendan did not decrease mortality or duration of intensive care unit or overall hospital stay [40]. Therefore, levosimendan should be used in selected children with ADHF who do not respond to standard vasoactive medications.
5. Mechanical circulatory support
Extracorporeal membrane oxygenation (ECMO) and ventricular assist devices (VADs) are 2 examples of cutting-edge technologies used to manage HF.
6. Extracorporeal membrane oxygenation
Over the last several decades, individuals with CHD who underwent surgical repair have been increasingly treated with ECMO for cardiopulmonary failure. However, ECMO should only be considered when all other medical options are exhausted. Rapid ECMO deployment, often known as “E-cardiopulmonary resuscitation,” has been developed in recent years as emergency life support for patients with cardiac arrest who are unresponsive to standard cardiopulmonary resuscitation. Despite major advancements in ECMO technology and management, in-hospital mortality associated with ECMO remains high along with other serious risks, including cerebral bleeding, sepsis, and kidney failure [41,42].
7. Ventricular assist devices
VADs play a crucial role in the management of selected patients with severe HF awaiting heart transplantation. They help maintain adequate systemic pressure and output to allow end-organ perfusion and function while unloading the failing ventricle, lowering myocardial oxygen demand, and encouraging favorable remodeling (especially in the brain, heart, and kidneys) [42]. Although modern implanted VADs are widely available for adults, only a few are available for children. The timing of VAD implantation and patient selection are critical for successful outcomes. In a prospective clinical study of VAD implantation in children, the VAD support group had considerably higher survival rates than the ECMO control groups [42]. The first pediatric VAD quality improvement network (Advanced Cardiac Therapies Improving Outcomes Network [ACTION]) was created in 2007. A recent ACTION analysis showed excellent VAD outcomes, including 96% 1-year survival, while the most common adverse events were major bleeding and infection [43].
8. Newer drugs for ADHF
Many other drugs have shown promising results in adult HF trials; however, similar evidence is currently unavailable to recommend or refute their use in the management of PHF. Such drugs include serelaxin, istaroxime ularitide, and recombinant human relaxin-2, which act as vasodilators and maintain end-organ perfusion. In the RELAX-AHF (Relaxin for the treatment of acute heart failure) study, serelaxin significantly reduced HF symptoms and mortality in adults hospitalized for AHF [44]. However, a similar pediatric trial (RELAX-PEDS-PK) was terminated early after disappointing findings from the RELAX-AHF-2 study in adults [45].
Istaroxime was the first synthetic medication with inotropic and lusitropic actions comparable to those of Digitalis. The HORIZON-HF (Hemodynamic, Echocardiographic, and Neurohormonal Effects of Istaroxime, a Novel Intravenous Inotropic, and Lusitropic Agent: a Randomized Controlled Trial in Patients Hospitalized with Heart Failure) trial demonstrated improved diastolic function and pulmonary capillary wedge pressure (PCWP) in adult patients with HF; however, no pediatric studies are currently available [46]. Synthetic natriuretic peptides (ularitides) stimulate the guanylate cyclase/cyclic guanosine monophosphate pathway, resulting in increased natriuresis, diuresis, and vasodilation. Clinical studies of ularitide in adults with ADHF indicated a reduction in PCWP and dyspnea [47]; however, no pediatric studies are available on its safety or efficacy.
Chronic heart failure
Children with CHF should be managed appropriately to promote early recovery, prevent progression, and correct the underlying causes, such as CHD. ACEIs, ARBs, diuretics, aldosterone antagonists, and β-blockers are commonly used in different combinations for patients with HFrEF. No treatment has been shown to reduce mortality and morbidity in HFpEF; however, diuretics are frequently used to reduce congestion and the associated symptoms. Although many adult trials have shown good safety and efficacy for these drugs, few pediatric trials have demonstrated the same. Thus, current pharmacological management options are mainly guided by the results of studies on adults (Table 3).
Drugs for managing CHF
1. Angiotensin-converting enzyme inhibitors
ACEIs have a class 1 (level of evidence, B) indication for individuals with LV dysfunction and a class IIa recommendation for asymptomatic individuals [3,4]. Patients with HF benefit from ACEIs because they alleviate symptoms, decrease HF progression, reduce the need for hospitalization, and increase survival by inhibiting RAAS activation and adrenergic activity. However, large-scale RCTs on these medications in children are not available [4,10,48].
2. Angiotensin receptor blockers
As in adults,ARBs are used in children who are intolerant to ACEIs. A prospective randomized placebo-controlled study of 5,000 adult patients with HF reported that valsartan substantially improved EF, signs and symptoms of HF, and quality of life [3,4,10].
3. β-blockers
β-Blockers have a class IIa recommendation for initiation in children with symptomatic LV systolic failure. They inhibit chronic SNS activation in patients with HF and reverse LV remodeling by decreasing its dilatation and improving its systolic function [10,49]. Improved coronary perfusion, cardiac output, and avoidance of arrhythmia are additional benefits of β-blocker usage [3,4]. Although adult HF trials have shown survival benefits with selective blockers, similar results have not been reported in children. A multicenter RCT failed to show the benefits of carvedilol over a placebo in children with HFrEF; however, this could have been an underpowered study with a large placebo effect [50]. A Cochrane review, including 7 studies with 420 children receiving β-blockers for HF, suggested a beneficial role of β-blockers in managing PHF [49].
4. Diuretics
Loop diuretics (e.g., furosemide) are the preferred drugs to treat HFpEF and achieve euvolemia. However, a combination of diuretics is commonly used to reduce side effects. There is a class I (level of evidence, C) recommendation for the use of aldosterone antagonists in patients with PHF associated with LV dysfunction [10,48].
5. Digoxin
The use of digoxin in PHF is mostly empirical and based on findings of adult studies. Recent guidelines recommend against the use of digoxin in children with asymptomatic LV dysfunction, as adult trials have not demonstrated survival benefits (class I; level of evidence, C). However, it can be used in symptomatic patients with low PHF levels (class IIa; level of evidence, C). Digoxin is also helpful in children with severe HF intolerant to ACEIs and β-blockers and in children with CHD and HF to reduce symptoms and hospitalizations [4,10,48].
6. Ivabradine
Ivabradine reduces the heart rate by decreasing the rate of phase-4 depolarization in the sinoatrial tissue, which is controlled by voltage. In a retrospective analysis of patients with end-stage Duchenne cardiomyopathy, ivabradine reduced the occurrence of serious adverse events by reducing the heart rate [51]. In a small trial, ivabradine alleviated HF symptoms in children by lowering the heart rate as a direct consequence of the termination or decrease in inappropriate tachycardia caused by atrial automatic tachycardia [52]. Ivabradine has receivedU.S.Food and Drug Administration (FDA) approval for use for HF symptoms in children older than 6 months [35].
7. Sacubitril/valsartan
Sacubitril is the first medication that effectively blocks both angiotensin II and RAAS activity. Neprilysin physiologically degrades natriuretic peptides; hence, it plays a critical role in the cardiovascular system. In the landmark PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial, the sacubitril/valsartan combination was more effective than enalapril in reducing mortality and length of hospital stay in adults with HFrEF [53]. The relative risk of hospitalization or death due to major cardiac events was 18% lower in patients administered sacubitril/valsartan than in those administered enalapril. However, hypotension was more common with this combination than with enalapril despite the decreased occurrence of increased creatinine or serum potassium levels.
Newer drugs for CHF
Many new drugs have shown promising results in trials conducted in adult patients with HF; however, these are not yet recommended for managing PHF owing to the lack of pediatric trials. Omecamtiv/mecarbil increased the activity of cardiac myosin ATPase while activating cardiac myosin. The GALACTIC-HF (Global Approach to Lowering Adverse Cardiac Outcomes through Improving Contractility in Heart Failure) study showed a decrease in HF events and death in adult symptomatic participants receiving omecamtiv/mecarbil versus placebo [54]. Vericiguat, a new orally soluble medication, has been approved for the treatment of adult patients with HFrEF by modulating endothelial activities in cardiac and vascular smooth muscles through guanylyl cyclase enzyme activation.The VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) study demonstrated significantly decreased overall mortality, cardiovascular mortality, and hospitalization owing to HF in patients receiving vericiguat [55]. SGLT-2 inhibitors such as dapagliflozin and empagliflozin act on the proximal renal tubules to treat type II diabetes mellitus in adults. Recently, McMurray et al. showed that dapagliflozin improved survival in adult patients hospitalized with HFrEF with or without diabetes mellitus [56].
Cardiac resynchronization therapy
Cardiac resynchronization therapy (CRT) is recommended in adults with HFrEF (LVEF <35%) who do not respond to optimal HF medicines and have a QRS duration >120 msec. CRT improves HF symptoms by improving cardiac motion, reducing the risk of sudden death, and improving the quality of life in these patients. Its use is recommended in selected pediatric patients (especially adolescents and young adults), such as patients with systemic ventricles and a history of severe LV dysfunction (LVEF <30%), with or without associated ventricular arrhythmias secondary to CHD (aortic or mitral stenosis). For CRT in children, the cutoff value of QRS duration was taken as the 98th percentile for age [10].
Future Directions
Despite their interpretative limitations, recent data have shown that cardiac biomarkers may be useful for diagnosing and prognosticating myocarditis, CHD, cardiac surgery, and HF as well as assessing the severity and cardiac involvement in immune-related and other systemic illnesses. However, most studies discussing the association between elevated cardiac biomarker levels and clinical outcomes have been retrospective. Prospective trials demonstrating the usefulness of biomarkers in improving clinical outcomes by choosing appropriate treatments and reducing hospitalization are lacking. These studies are difficult to accomplish in the pediatric population because of the low patient frequency; however, they are urgently required to support evidence-based recommendations and appropriate clinical adoption of chosen cardiac biomarkers in the evaluation of cardiac and noncardiac diseases [57]. Adult HF findings may aid the design of appealing drug trials for pediatric patients, but adult trials cannot take their place. The underlying pathophysiology of HF and the pharmacological mechanisms of various drugs must be considered when choosing medicines for PHF. The pharmaceutical industry has little motivation to develop child-specific HF treatments owing to the low number of PHF cases. Governments can fund such trials and fill the knowledge gaps in the literature to accelerate pediatric HF drug and device studies. Novel clinical trial designs that accelerate the development, evaluation, and review of treatments for PHF and connect funding from the Centers for Medicare and Medicaid Services to FDA marketing clearance may enable early market access. Device-based treatments for children may reduce adverse medication effects and enhance HF treatment compliance rates [58].
Over the last several decades, PHF therapy has evolved to meet new standards of care and address new problems. Newer treatment options extend the lives of children with HF, but at a higher total cost; thus, preventive measures and high-quality care are urgently needed. Heart transplantation is the only viable alternative for preserving life because of its palliative nature and the short life expectancy in treatment-refractory HF. Assuming that cardiac dysfunction and its treatment are not restricted to heart rate (rhythm), myocardial contractility, preload, or afterload, effective but underutilized therapeutic options are available. Modifying the ventricular afterload by enhancing the contralateral ventricular performance represents a paradigm change in the management of PHF. The therapeutic use of adverse ventricular-ventricular interactions (VVI)is possible. Functional recovery may be accomplished in >80% of newborns with DCM despite the requirements for listing for orthotopic heart transplantation when surgical implantation of a pulmonary artery banding is used to restore heart function through VVI [58].
Conclusion
Recent advances in diagnostic and therapeutic approaches to PHF have significantly changed outcomes. Newer diagnostic approaches such as cardiac MRI, speckle-tracking echocardiography, tissue Doppler imaging, and molecular techniques have increased our understanding of PHF. The findings of recent adult HF trials have suggested the possibility of applying newer drugs such as ARNIs and SGLT-2 inhibitors for managing PHF. However, further pediatric trials are needed to establish the efficacy and safety of these drugs in children considering the differences in the underlying pathophysiology of PHF in pediatric versus adult patients as well as the mechanism of action of different drugs. Future outcomes for children with HF will improve with the implementation of a multidisciplinary approach.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: DJ, AA, Data curation: DJ, AAAAZ, ATE, AA, Formal analysis: DJ, AAA AZ, ATE, Writing - original draft: DJ, AAAAZ, ATE, AA, Writing - review & editing: AA