Practical issues of oral immunotherapy for egg or milk allergy
Article information
Abstract
Oral immunotherapy (OIT) has been recommended to reduce parental burden related to strict allergen avoidance and induce desensitization and immune tolerance for patients with long-lasting allergies to hen’s eggs (HE) or cow’s milk (CM). OIT should be monitored by pediatric allergists specializing in OIT and oral food challenge tests to manage allergic reactions. Although a previous history of anaphylaxis or multiple food allergies is not a contraindication to OIT, it is contraindicated if the patient has uncontrolled asthma, a malignancy, active systemic autoimmune disorders, or diseases requiring treatment with beta-blockers. A variety of OIT protocols have been de veloped to ensure better outcomes and safe up-dosing, including adjunctive therapies with biologics. This review provides insight into the practical issues of various immunotherapy options for children with HE or CM allergies.
Key message
· Oral immunotherapy should be supervised by pediatricians with experience administering oral food challenge tests and managing allergic reactions.
· Food allergen intake is gradually increased and maintained for years.
· Patients may experience allergic reactions and psychological problems.
· Adjunctive therapies (biologics, antihistamines, and leukotriene receptor antagonists) may improve efficacy and safety.
· Contraindications include uncontrolled asthma, malignancy, active autoimmune disorders, and beta-blocker usage.
Graphical abstract.
Introduction
Food allergies (FAs) are an increasing public health problem in childhood, with a worldwide prevalence of approximately 3%–6% [1,2]. In Korean schoolchildren, the prevalence of immediate-type FAs in 2015 was reported to be 3.2% in 6- to 7-year-olds, 4.5% in 9- to 10-year-olds, 4.0% in 12- to 13-year-olds, and 4.5% in 15- to 16-year-olds [1]. Hen’s egg (HE) and cow’s milk (CM) are the most frequent causative food allergens in Korean children younger than 18 years [1,3-5]. The public health burden of FAs has become important, as it can cause major quality of life (QoL) impairments for patients and their families [6,7]. Indeed, young patients and their parents who have experienced anaphylaxis are more likely to develop psychiatric diseases such as posttraumatic stress disorder, anxiety, and depression [8,9]. Previous studies reported that families of children with FAs incurred additional annual direct and indirect household costs of up to $2,500 and $1,800 compared to families with healthy children [6].
Although strict allergen avoidance is the only way to prevent food-induced allergic reactions, it is difficult for caregivers to avoid eggs and milk, which are present in a variety of processed foods [10]. The restriction of these protein sources in the diets of children and adolescents negatively affects their health status [2,11]. In our recent study, only half of the Korean children allergic to CM or egg whites (EWs) had outgrown their allergies by 8.7 and 5.6 years of age, respectively [12]. Given these difficulties, oral immunotherapy (OIT) has emerged as an active treatment option to induce desensitization and immune tolerance in patients with long-lasting allergies to HE or CM [13]. This review highlights the current knowledge and future perspectives on various immunotherapy strategies in children with immunoglobulin E (IgE)-mediated HE or CM allergies.
OIT preparations
Recent guidelines have recommended OIT for children who have IgE-mediated FAs and place a high value on their ability to eat offending foods under the supervision of a specialist from around 4–5 years of age [14-17]. To ensure OIT safety and success, an accurate diagnosis of IgE-mediated FAs through the detection of specific IgE antibodies and oral food challenge (OFC) tests should precede OIT [18]. An OFC is also recommended to determine the baseline threshold of offending foods, determine the degree of cooking required, and assess desensitization or sustained unresponsiveness (SU) after OIT [19]. Although double-blind placebo-controlled OFC tests are the gold standard for diagnosing FAs, an open challenge test with a 4- or 6-dose protocol can be used in children to confirm FAs due to the low possibility of bias and psychological effects [19,20]. The OFC dose can be set depending on the purpose, such as confirming FAs or determining the initial OIT dose. Desensitization and SU were regarded when there was no reaction during OFC testing with a target dose after a build-up phase and after at least 2–4 weeks of avoidance following maintenance therapy, respectively [21,22].
OIT should be supervised by specialized pediatricians with experience conducting OFC tests and managing allergic reactions [20]. Before the beginning of OFC and OIT, informed consent should be obtained from the patient’s guardians after sufficient information is provided on the process, potential outcomes, benefits, and risks of OIT. Hospitals should be equipped with drugs and facilities for possible symptoms associated with OFC tests or OIT. The individualized OIT schedule should be provided to the patient’s caregivers in clear and simple documents [14].
It is necessary to prepare protocols for visiting hospitals according to the situation of each country or center, as access to medical care and financial burden in the OIT process may differ among countries [23]. In addition, the types and cooking methods of food consumed during OIT contribute to the protocol’s efficacy and adherence [16]. For example, allergen pancakes and shepherd’s pie, mainly eaten in the United Kingdom to treat CM allergies, are unfamiliar to Koreans [16]. Boiled eggs, which are easy to prepare at home, may not be able to induce SU or oral tolerance [24,25]. Although OIT with inpatient and outpatient management has been conducted at several hospitals in Korea, there is a large variance among OIT protocols. Therefore, as reported in other countries such as France, Canada, Spain, and Japan, evidence-based guidelines are needed to ensure OIT safety and efficacy [14,15,20,26,27].
Immunological changes during OIT
As the amount of food consumed increases during OIT, allergen exposure leads to T helper 2 (Th2) and allergen-specific Th2 anergy, as well as an increase in regulatory T (Tregs) and interleukin-10-producing CD4+ T cells, which reduce the production of specific IgE and increase levels of allergen-specific immunoglobulin A and immunoglobulin G (IgG)4 [28]. Circulating allergen-specific IgG neutralizes allergens, while IgG bound to the cell surface FcγRIIb induces inhibitory signaling with IgE and IgG crosslinking, preventing mast cell and basophil degranulation in desensitized patients [29]. Decreased wheal size on skin prick tests that occurs several months after OIT is related to basophil hyporesponsiveness [11,30]. A recent study using single-cell RNA-seq and paired T-cell receptor α/β sequencing demonstrated that better OIT outcomes were associated with stronger suppression of Th2 module expression in Th2A-like cells. At the same time, an association was noted between treatment failure and the expression of inflammatory gene signatures in Th1 and Th17 cells [31]. Further studies are needed to elucidate the mechanism by which OIT affects the ability to achieve SU at the cellular level.
Conventional OIT
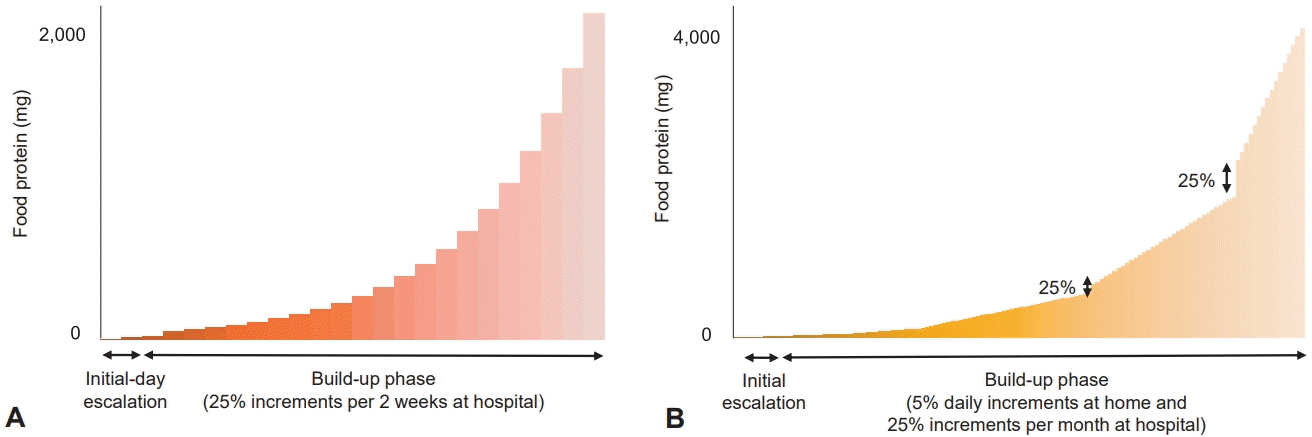
OIT consists of an initial escalation, a build-up phase, and a maintenance phase in which the daily intake of food allergens is maintained for several months or years [13]. Initial dose escalation is performed on days 1–3, beginning with an extremely small dose rapidly increasing (usually from 0.01 to 0.1 mg of protein) and remaining at subthreshold levels to identify dose safety [11,30,32]. During the build-up phase, the increases in the allergens, according to various protocols, are conducted under a physician’s supervision by up to 25%–100% every 1–2 weeks until the target maintenance dose (300 to 4,000 mg of protein) is achieved [23,32,33]. Burks et al. [30] first evaluated OIT using EW powder to treat children with HE allergies in a double-blind, randomized, placebo-controlled study. After 10 months, none of the children in the control group versus 55% in the OIT group passed the OFC; after 22 months, 75% of the children in the OIT group were desensitized.In the OIT group, 28% passed the OFC at 24 months and were considered to have achieved SU. HE OIT induced desensitization and tolerance in 35%–94% and 28%–78% of patients with HE allergies, respectively [30,34-36]. Similarly, CM OIT resulted in desensitization and tolerance in 37%–100% and 23%–45% of patients allergic to CM, respectively [22,37,38].
Adverse reactions
Although OIT is known to induce desensitization or SU in patients with FAs, 30%–80% of patients experience adverse reactions such as mucocutaneous symptoms, bronchospasm, abdominal pain, vomiting, and anaphylaxis during the build-up phase (Table 1) [25,36,39,40]. The adverse rates per dose were 6.5%–31%, while the adverse rates per patient were 30%–100% [34,39-41]. Symptoms usually manifest during the build-up phase, but they may also arise during the maintenance phase. Additionally, OIT-related adverse reactions can be triggered by various factors, including exercise, bathing, acute infections, and psychological stress. Eosinophilic esophagitis (EoE) has been reported in 1%–2.7% of patients who undergo OIT for allergies to HE, CM, or peanuts [42,43]. Symptoms are often nonspecific and may include abdominal discomfort, pain, regurgitation, and vomiting [42]. Therefore, suspicion and a careful evaluation by a gastroenterologist, including an esophagogastroduodenoscopy and biopsy, are necessary to ensure a proper diagnosis and an appropriate therapeutic plan. It remains unclear which patients are at increased risk of developing EoE during OIT, and further research is needed to identify the risk factors and assess the prognosis of EoE.
Some patients experience psychological stress and anxiety due to repeated adverse reactions, influencing their motivation for and clinical outcomes after OIT [30,32]. A recent study showed that more than 80% of patients sought psychological support for emotional problems and eating difficulties during the initial and build-up phases of OIT [44]. Therefore, clinicians should be aware of patients’ emotional problems and provide psychological support to help them cope with OIT-related difficulties and improve their treatment adherence. The anxiety levels of patients and caregivers may temporarily increase, but previous studies reported that OIT eventually improves caregiver QoL compared to baseline levels [45,46]. The QoL of caregivers improved significantly when the maintenance phase was reached and for 6 months thereafter [46]. Therefore, OIT can help reduce the psychological burden in the absence of psychological problems severe enough to make it difficult to maintain long-term OIT [45].
The action plan for patients and caregivers includes monitoring for possible side effects, managing adverse reactions, observing for treatment sequelae, and planning subsequent OIT schedules. Additionally, bidirectional communication is needed to enable patients or caregivers to participate in the decision-making process and consult an allergist with any questions or suggestions during OIT [18,23]. Most of the adverse reactions were mild and self-limited, and there were no reports of OIT-related deaths; however, up to 20% of patients discontinued OIT due to frequent allergic reactions and serious anxiety [30,41].
Home-based up-dosing and modified immunotherapy protocols
Various OIT protocols have been reported to ensure safe up-dosing at home to overcome the inconvenience of conventional protocols that frequently feature adverse reactions and require hospital visits during the build-up phase (Table 2). Slow OIT protocols with a maintenance dose much lower than the full dose have been developed for children with severe FAs to ensure safety and avoid accidents due to hidden allergen exposure [47]. In our previous study, children in the OIT group increased the amount of boiled EWs by 5% per day at home and 25% per month at hospitals, with a target dose of 4.0 g of boiled EW proteins (Fig. 1) [48]. After completing a 5- to 13-month build-up phase, 93.8% (15 of 16) of patients with OIT showed desensitization, while only 6.2% (1 of 16) of patients in the control group passed OFC tests. Adverse reactions occurred in 75.0% of patients with OIT, including 25% of anaphylaxis cases. Notably, no patients demonstrated serious anxiety or life-threatening events, the greatest obstacles to maintaining OIT. A Spanish clinical trial that included home-based OIT using pasteurized EWs showed an 84.2% desensitization rate and a 90.8% adverse reaction rate in patients with OIT [36].
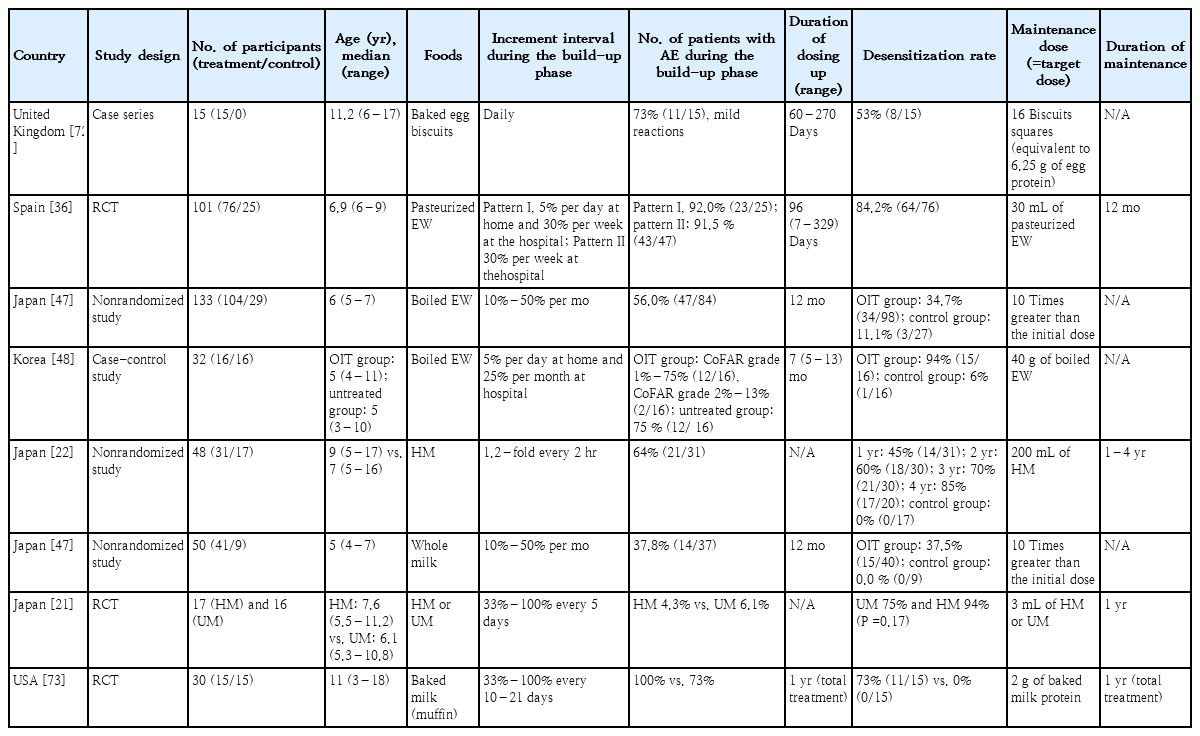
Summary of recently published studies of modified oral immunotherapy protocols for managing egg or milk allergy
CM OIT is also associated with frequent adverse reactions that lead to treatment discontinuation [49,50]. Therefore, modifications to the OIT protocol have been explored, such as adjustments to the speed and degree of up-dosing and processing of the consumed food itself. OIT protocols using heated or baked milk have been developed for safe up-dosing, as the heating or baking process reduces allergenicity by destroying conformational epitopes [51]. Recent studies demonstrated that 60%–80% of children with IgE-mediated CM or HE allergies tolerate baked milk or eggs [18,52]. However, the baked milk OIT protocol showed a low desensitization rate of <70% for unheated milk (UM), which did not eliminate concerns about CM exposure in real life despite less frequent adverse reactions of 8%–33% [53-55]. To address these issues, Takahashi et al. [22] used CM heated in a microwave oven for OIT. Once the volume of heated milk (HM) reached 200 mL, patients consumed 200 mL of HM daily for 2 months at home, then shortened the time to heat the CM in the microwave, and then switched to 200 mL of UM. After a 2-week off-treatment period, 22.6% ofthe subjects passed the OFC test. Another Japanese study conducted OIT with a small amount of CM (3 mL) after randomization into HM and UM in patients >5 years of age with a CM allergy [21]. After 1 year, 18% of patients in the HM group versus 31% in the UM group passed the 25 mL OFC test, showing no statistically significant difference [21]. On the other hand, the incidence of moderate to severe adverse responses was significantly lower in the HM group (0.7%) than in the UM group (1.2%) during OIT, which can induce immunological changes more safely when HM is used on the OIT compared to UM [21,47,11,56-58].
Use of adjunctive therapies during OIT
Adjunctive therapies with biologics, ketotifen, and leukotriene receptor antagonists (LTRAs) have been developed to improve the efficacy and safety of OIT by blocking allergic reaction downstream effects by targeting IgE or mast cell mediators [29,59,60]. Above all, omalizumab has been used as an adjuvant during the dose escalation period to reduce adverse reactions and improve efficacy. For example, Wood et al. [61] showed that the omalizumab group took less time to reach the maintenance phase, although the desensitization rate and SU did not differ significantly from those of the CM OIT alone group. A recent real-world study conducted in Spain investigated 58 children with severe CM allergies treated with omalizumab [62]. In that study, 83% of patients tolerated ≥6,000 mg of CM protein during the maintenance phase, while 40.5% of patients who completed follow-up tolerated CM without omalizumab. Notably, anaphylaxis occurred in 36.4% of patients who discontinued omalizumab, with a higher incidence in those who discontinued suddenly (50.0%) versus gradually (12.5%) [62]. It was recently suggested that calculating the dosage of omalizumab per body weight yields better clinical outcomes during the initial escalation phase than using the standard dosage per weight and total IgE levels [63]. Additionally, ketotifen and LTRAs were reported to prevent adverse reactions, especially gastrointestinal symptoms, during OIT with HE, CM, wheat, or peanuts in previous studies [64,65]. Studies of other biologics have also been conducted, such as the use of dupilumab (NCT03793608) or anti-interleukin-33 (NCT0290021) during peanut OIT, raising expectations for their use in HE or CM immunotherapy [66].
Recommendations and contraindications for OIT
OIT is a treatment in which the food allergen, which had been strictly restricted for fear of serious allergic reactions in the past, is ingested regularly; therefore, it requires careful supervision according to the predetermined schedule due to the possibility of adverse reactions [18,23]. Patients and their caregivers should be prepared to recognize and manage allergic reactions during OIT. However, a previous history of anaphylaxis to the targeted food or multiple FAs is not a contraindication to OIT [17]. Patients should not take allergens on an empty stomach or go to bed within 1–2 hours after their administration [16]. Additionally, hot showers or baths, physical exertion, infection, gastrointestinal disease, dental procedures or surgeries, menstruation, lack of sleep, and uncontrolled underlying allergic diseases may increase the likelihood of adverse reactions during OIT [14,32].
Consensus is lacking on the best age for OIT, but it is challenging to start in infancy due to limitations in the expression of symptoms that may occur during treatment [17]. Although guidelines suggest that OIT can be considered from around 4–5 years of age, the age at which OIT is started may depend on the patient’s developmental status, family situation, allergic reaction severity, and risk-benefit ratio [14,16]. To ensure OIT safety, weak motivation, poor adherence to instruction, reluctance to use medications, severe anxiety, language barriers, and psychiatric problems should be evaluated before its commencement [17,44]. OIT is contraindicated if the patient has uncontrolled asthma, malignancy, active systemic autoimmune disorders, or diseases requiring beta-blocker usage (Table 3) [14,23]. Relative contraindications include active severe atopic dermatitis, EoE, eosinophilic gastrointestinal diseases, mastocytosis, and heart disease [17]. During OIT, gastrointestinal symptoms should be monitored, as an increase in IgG4 and activated allergen-specific Th2 cells may affect the development of EoE [67].
Future directions in studies of food OIT
According to the results of studies in patients with IgE-mediated peanut allergy, elevated concentrations of IgG4 and low levels of IgE to Ara h 2 were found in patients who acquired immune tolerance, whereas high levels of Ara h 2-specific IgE and Th2A cells were associated with persistent allergies [66,68]. A recent study demonstrated that the possibility of tolerance is likely higher in young children with a low specific IgE, a high threshold, and no experience of anaphylaxis, even with shorter OIT periods at small doses, suggesting that the immune and clinical phenotypes of FA may be related to immunotherapy outcomes [69]. However, few studies have examined which subgroup responds well to OIT, although biomarker identification and subgroup analysis may provide better individualized FA treatment options. Moreover, the OIT protocol must be standardized, and more research must be conducted investigating the mechanisms and long-term effectiveness of food immunotherapy. Given these gaps, questions remain regarding the time needed to reach tolerance and appropriate biomarkers of OIT. Not surprisingly, treatment of multiple FAs is of growing interest because approximately 30% of children with FAs suffer from allergies to more than one food, and single-food OIT does not significantly improve QoL [70,71]. Further clinical trials are needed to assess the safety and efficacy of multi-OIT protocols for patients with multiple FAs.
Conclusion
Children with HE or CM allergies are at risk of nutritional deficiencies and psychological problems. OIT could be a safe and effective option to induce desensitization in patients with FAs. However, the risk of adverse events during OIT remains a concern. Thus, a personalized action plan should be provided to patients and their caregivers to treat possible allergic reactions. The patient’s inability to cope with protocol, uncontrolled asthma, and psychiatric barriers should be assessed before OIT initiation. Recent studies suggest that many promising adjunctive therapies might help patients optimize OIT administration. However, the discontinuation of omalizumab can be associated with severe allergic reactions during OIT and should be carefully monitored. Unfortunately, there is a paucity of literature regarding clear indications for OIT and standard protocols according to clinical and immunological characteristics. Thus, further studies should determine which specific phenotype of patients with FAs would benefit the most from OIT as well as which therapeutic options or dosing schedules could be applied to achieve immunotolerance.
Notes
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
This study was supported and funded by the Kunhee Lee Child Cancer & Rare Disease Project, Republic of Korea (grant number: FP-2022-00001-011).
Acknowledgements
The authors thank Chanmi Moon from Multimedia Services Part, Samsung Medical Center (Seoul, Republic of Korea) for drafting the graphical abstract.