Prevalence, risk factors, and treatment of small intestinal bacterial overgrowth in children
Article information
Abstract
Small intestinal bacterial overgrowth (SIBO) is defined as the presence of an excessive number of bacteria within the small bowel. Pediatric SIBO is a heterogeneous disorder that manifests as various symptoms ranging from mild gastrointestinal symptoms to malabsorption or malnutrition. The carbohydrate breath test is a commonly used, safe, and noninvasive diagnostic test; however, a standardized methodology is lacking. Multiple factors, such as neuromuscular disorders, systemic diseases, chronic drug use, or altered intestinal anatomy that disturb intestinal motility or induce an abnormality in the body’s defense systems against intestinal bacteria, predispose children to SIBO. The high prevalence and similar symptoms of SIBO in functional gastrointestinal disorders, including irritable bowel syndrome, suggest an association between them. The principles of treatment include managing predisposing conditions, nutritional support, symptom control, and antibacterial treatment. Rifaximin is the most commonly used drug. To date, studies of antibiotic treatment in pediatric populations with irritable bowel syndrome or SIBO are lacking and have shown mixed results. Here we review the prevalence, diagnostic tests, and treatment results in pediatric populations.
Key message
· Pediatric small intestinal bacterial overgrowth (SIBO) manifestations range from nonspecific abdominal symptoms to malabsorption or malnutrition.
· SIBO is prevalent in children and adolescents with functional abdominal pain disorders.
· Predisposing factors include disturbed intestinal motility, altered anatomy, and/or abnormal body defense systems against intestinal bacteria.
· Breath tests are safe and noninvasive.
· Treatment principles include managing predisposing conditions, nutritional support, symptom control, and antibiotics.
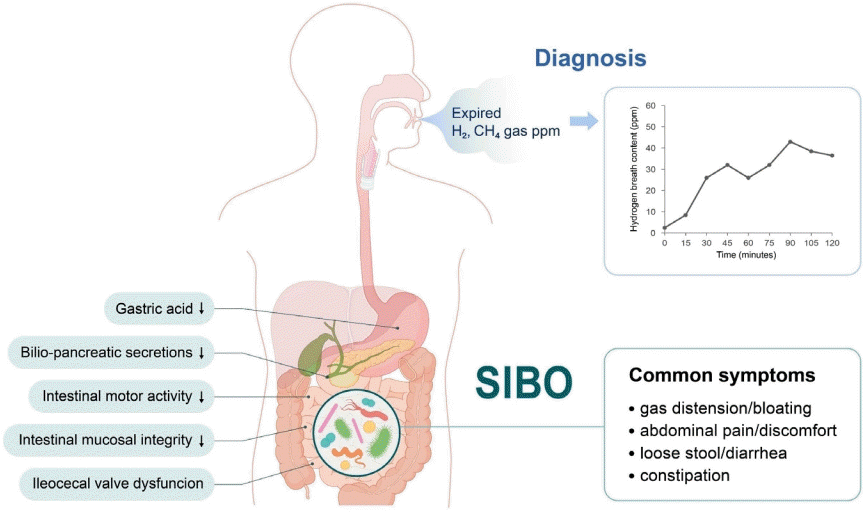
Graphical abstract. SIBO, small intestinal bacterial overgrowth; H2, hydrogen; CH4, methane.
Introduction
Small intestinal bacterial overgrowth (SIBO), defined as an excessive number of bacteria within the small bowel, exhibits various gastrointestinal symptoms, particularly when it involves bacteria not commonly found in the small intestine [1]. Pediatric SIBO is a heterogenous disorder that manifests as broad symptoms ranging from mild abdominal pain to malabsorption and malnutrition [2]. Its most common symptoms are chronic abdominal pain, abdominal distention, diarrhea, flatulence, belching, steatorrhea, fetid stools, mucus in the stools, fatigue, nausea, and stunted growth [1].
Definition of SIBO
SIBO is defined as specific or nonspecific gastrointestinal symptoms caused by an excessive bacterial burden within the small bowel. The bacterial load in the small intestine can be directly measured by quantitative culture of small bowel aspirates, while it can be indirectly measured by the amount of exhaled hydrogen gas in the breath after the ingestion of carbohydrate substrates. Bloating is the most common symptom of SIBO. Other symptoms include nausea, abdominal distention, cramps or pain, flatulence, diarrhea, and/or constipation. Irondeficiency anemia, fat-soluble vitamin B12, and vitamin D deficiencies are uncommon and usually seen in patients with postsurgical anatomical changes or blind loop syndrome. Severe symptoms include steatorrhea and weight loss. The 2020 American College of Gastroenterology (ACG) [3,4] or European guideline [5] recommends the use of hydrogen (H2) breath testing for the diagnosis of SIBO in patients with irritable bowel syndrome (IBS), in symptomatic (abdominal pain, gas, bloating, and/or diarrhea) patients with suspected motility disorders, and/or those with a previous history of luminal abdominal surgery. Methane (CH4) using glucose or lactulose breath tests to diagnose the overgrowth of CH4-producing organisms in symptomatic patients with constipation has also been recommended [3,4,6]. The ACG suggests against the use of breath testing for the diagnosis of SIBO in asymptomatic patients taking proton pump inhibitors.
Orocecal transit time
SIBO can be caused by archaea or bacteria, single or multiple microorganisms, gram-positive or -negative bacteria, and anaerobic or aerobic microorganisms. The human body produces H2 and CH4 via intraluminal microbial fermentation and methanogenesis, respectively. Undigested carbohydrates are easily reached in the colon and fermented by H2-producing bacteria, resulting in physiological increases in H2 in the colon. In turn, intraluminal H2 is absorbed into the systemic circulation, transported to the lungs, and released into the breath. Thus, orocecal transit, defined as the time that elapses from ingestion to reaching the cecum, is about 90 min for both adults and children on the lactulose H2 breath test [1,3,6].
Diagnostic methods
Normally, the number of bacteria in the proximal bowel is approximately 102 colony-forming units (CFU)/mL, which gradually increases as it approaches the terminal ileum [1]. The abundance of numerous pathogenic organisms is increased in subjects with SIBO and IBS, including but not limited to Enterococcus, Escherichia coli, and Klebsiella. The gold standard for diagnosing SIBO is a jejunal aspirate culture with ≥103 CFU/mL. However, this is not always practical in pediatrics [3,4] because culturing is costly and requires invasive endoscopy. A patchy distribution of SIBO may cause false negativity; in contrast, it can be contaminated by the oral flora. Moreover, standardized methodologies are lacking. The carbohydrate breath test is a commonly used culture-independent test. Glucose and lactulose are commonly used substrates in breath tests.
Glucose is a monosaccharide that is easily absorbed by the proximal small intestine. In the presence of SIBO, glucose administration may result in an H2 peak within 90 minutes. The glucose breath test may not adequately detect SIBO in the distal bowel as it is readily absorbed in the proximal small intestine. An increase in orocecal transit time, as the glucose quickly reaches the colon and may undergo early fermentation, results in false positivity on the breath test. A false-negative result may also occur when there are no H2-generating bacteria or CH4-generating microorganisms [1,3]. Lactulose is an indigestible disaccharide that has limited absorbability, and it reaches the cecum in an undigested state. Lactulose can produce an initial peak owing to the excessive growth of bacteria in the small intestine and a second peak caused by colonic microbiota fermentation. The lactulose breath test is limited by its potential false-positive rate in highly motile patients. The sensitivity and specificity of the lactulose H2 breath test is 31%–68% and from 44%–100%, respectively.
Constipation is associated with elevated breath CH4 and stool Methanobrevibacter smithii test results. M. smithii, a member of the Archaea domain, has been linked to constipation-predominant IBS [6,7] and is the most abundant methanogen responsible for CH4 production. Because archaea are not bacteria, intestinal methanogen overgrowth (IMO) is more appropriate for treating M. smithii overgrowth [7]. Nearly 30% of the general population has IMO. Archaea use H2 to produce CH4; thus, H2 breath test results can be falsely negative, while CH4 breath test results can be falsely positive [7]. Therefore, testing for CH4 using glucose or lactulose breath tests to diagnose IMO concurrently with H2 is recommended to avoid false-negative results in symptomatic patients with constipation [3-7]. Studies in adults demonstrated that CH4 production is more prevalent in those with constipation [8]. In pediatrics, CH4 production is positively correlated with prolonged whole-intestinal transit time [9].
H2–CH4 breath testing remains a useful, noninvasive, and safe diagnostic tool for diagnosing SIBO in children. The breath test, compared with jejunal aspirate cultures, showed sensitivity of 60%–70% and specificity of 40%–80% for a SIBO diagnosis [4]. The sensitivity and specificity of the glucose H2 breath test was 20%–93% and 30%–86%, respectively. The North American Consensus [3] recommends that a SIBO diagnosis could be proposed if H2 increases by >20 ppm from the baseline or by >10 ppm within the first 90 minutes of the lactulose or glucose breath test. The Rome Consensus [10] recommends a glucose H2 breath test owing to its higher accuracy; for SIBO, a positive glucose breath test is defined as an H2 of ≥12 ppm compared to the reference value. The major limitations of diagnostic tests are the lack of standardization, substrates, or cutoff criteria [3,4,10]. Nevertheless, it is important to use defined doses of glucose (75 g) and lactulose (10 g) for standardization [3,4]. However, there is no suggested definition of SIBO for Asian or pediatric patients, so further consensus is needed.
Risk factors for SIBO
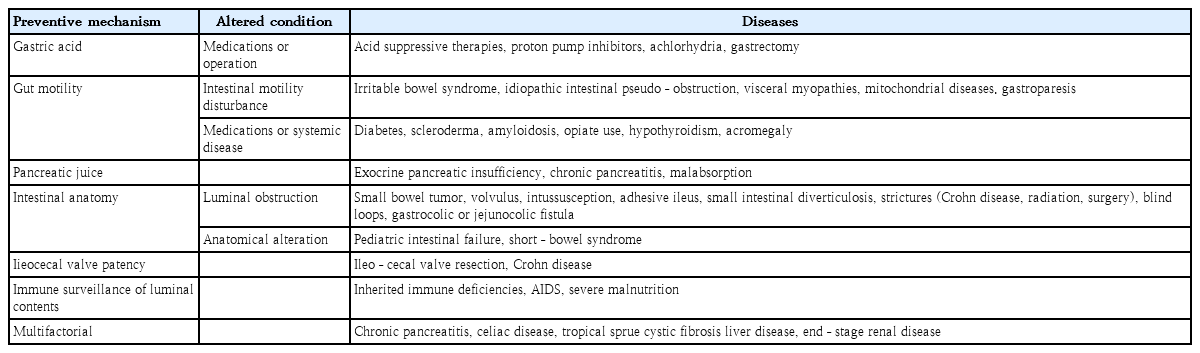
Disturbances in intestinal motility and/or abnormalities in the body’s defense systems against intestinal bacteria predispose children to SIBO (Fig. 1). These provocative conditions can cause SIBO. Several factors contribute to the maintenance of microbial homeostasis in the small intestine. The antimicrobial effects were created by gastric acid, bile, or pancreatic enzymes; adequate gut motility; normal intestinal anatomy; and the patency of the ileocecal valve to maintain homeostasis [2,7,10]. The migrating motor complex acts as an intestinal housekeeper that sustains the aboral progression of luminal content in the small intestine between meals, thereby preventing stasis. Absence or disruption of migrating motor complexes may lead to SIBO. Small bowel transit time was significantly slower in patients with versus without SIBO. Defective immune monitoring results from impaired gut motility and luminal stasis.
A patent ileocecal valve prevents backwash of the luminal contents of the colon into the small intestine. The mean ileocecal junction pressure was significantly lower in patients with SIBO. Surgical blind loops or anatomical alterations predispose children to SIBO [2,7]. Mechanical obstruction caused by small bowel tumors, volvulus, intussusception, or adhesive ileus predispose children to SIBO. Medications such as opiates and proton pump inhibitors and systemic diseases such as diabetes, scleroderma, amyloidosis, and malabsorptive conditions can be associated with SIBO (Table 1).
Prevalence of SIBO in IBS
Multiple pathophysiologic factors contribute the functional gastrointestinal disorders. Altered bowel motility, visceral hypersensitivity, abnormal brain-gut interactions, autonomic dysfunction, low-grade mucosal inflammation, and activation of mucosal immunity are risk factors for SIBO that can cause various symptoms of IBS. Although the prevalence of SIBO varies widely depending on test method and study size, it is reportedly approximately 2.5%–22% [2,7]. SIBO is generally underdiagnosed because it may be asymptomatic; if symptoms are present, they are usually nonspecific. Accordingly, patients are less likely to visit a medical institution or be diagnosed properly.
SIBO, one feature of gut dysbiosis, is a potential cause of symptoms in IBS patients [7]. SIBO occurs more often in IBS with diarrhea (IBS-D) than in those with IBS with constipation (IBSC) [7]. Seven of 16 epidemiological studies showed that the frequency of SIBO among IBS subjects was 4%–78% compared to healthy controls, of which only 1%–40% had SIBO [7,9,10]. A meta-analysis of 25 case-control studies (3,192 IBS subjects and 3,320 controls) showed that the prevalence of SIBO in IBS was 31.0% (95% confidence interval [CI], 29.4%–32.6%) with an odds ratio (OR) of 3.7 (95% CI, 2.3–6.0; P=0.001) compared to healthy controls or non-IBS patients [2,11]. Compared with only
healthy controls, the OR increased to 4.9 (95% CI, 2.8–8.6; P=0.001) [2,11]. The prevalence of SIBO in IBS subjects versus controls was 62.3% versus 33.5% for the lactulose breath test and 20.7% versus 4.4% for glucose. The association between SIBO and IBS was 35.5% (95% CI, 32.7%–40.3%) in IBS-D, 22.5% (95% CI, 18.1%–26.9%), or 25.2% (95% CI, 22.2%–28.4%) in IBS-C or mixed IBS [2,7,11].
Prevalence of SIBO in children
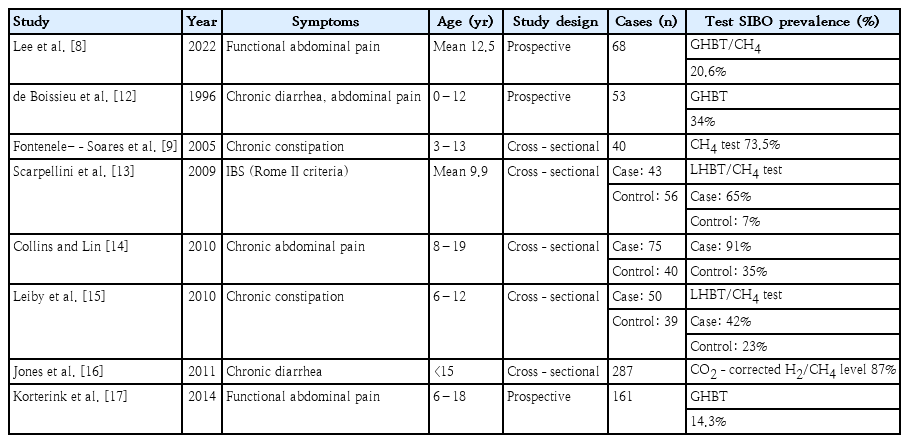
Despite fewer studies of SIBO in children versus adults, clinical studies have recently been published on a variety of pediatric patients. Symptoms of functional gastrointestinal disease and SIBO overlap. The various rates of prevalence of SIBO in children with chronic abdominal pain, constipation, chronic diarrhea, or IBS have been reported (Table 2) [8,9,12-17].
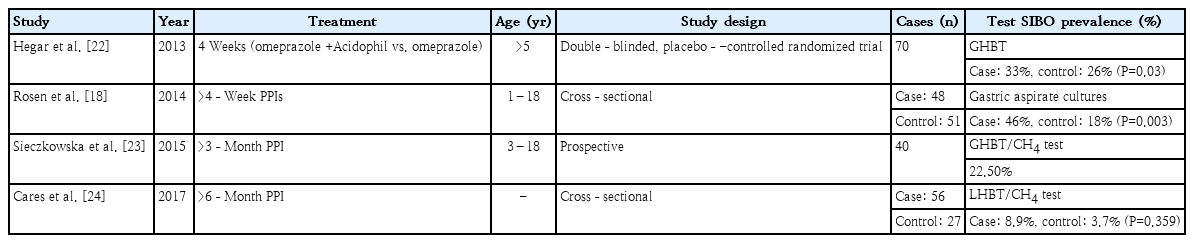
The use of proton pump inhibitors could predispose an individual to SIBO by lowering gastric acid levels and subsequently altering the intraluminal environment and bacterial flora [18,19]. However, several meta-analyses of adult populations showed consistent results [20,21]. A meta-analysis of 11 studies (3,134 patients) showed that the pooled OR of SIBO in PPI users versus non-users was 2.282 (95% CI, 1.238–4.205). An association between SIBO and PPI use was reported by studies that used duodenal or jejunal aspirate cultures to diagnose SIBO (OR, 7.587; 95% CI, 1.805–31.894), whereas no relationship was found between SIBO and PPI use in studies that used the glucose H2 breath test (OR, 1.93; 95% CI, 0.69–5.42) [21]. Future welldesigned case-control studies are needed to validate the diagnosis of bacterial overgrowth. Several prevalence studies in pediatric patients taking PPIs have been reported (Table 3) [18,22-24]. Thus, SIBO may be associated with environmental enteropathy and intestinal inflammation. Malnourished children in impoverished conditions are susceptible to SIBO. There are several reports that living in impoverished socioeconomic conditions is associated with SIBO in children (Table 4) [25-29]. However, one study of nutritional interventions failed to reverse the SIBO condition [30].
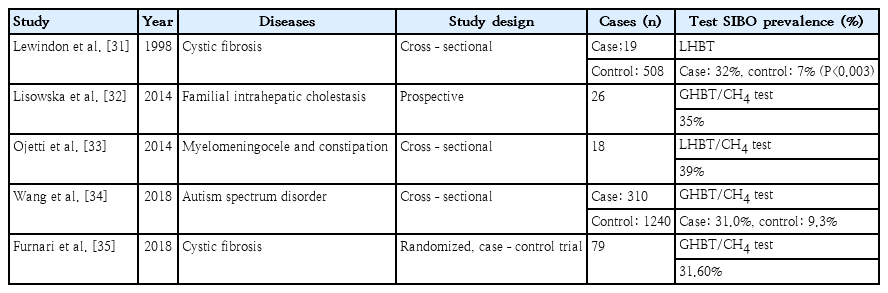
The prevalence of SIBO in children with various diseases was reported previously (Table 5) [31-35]. Data on the epidemiology of SIBO in children are limited by a lack of controls, variations in test methodology, and varying diagnostic cut-offs used [2]. Most research to date includes cross-sectional studies of small numbers of patients. SIBO may be positive in healthy controls with an estimated incidence of 0%–35% in healthy children [2]. The number of healthy subjects with SIBO likely suggests the possibility that a balance of specific bacterial strains with different adaptive host factors may produce asymptomatic or mildly symptomatic patients with SIBO [2]. This demonstrates the complex relationship between SIBO and IBS symptoms.
Lee et al. [8] recently reported the prevalence of SIBO in 68 children with functional abdominal pain disorders (FAPDs) using the Rome IV criteria using the glucose H2–CH4 breath test in Korea. SIBO was detected in 14 patients (20.6%). To our knowledge, this is the first study of the prevalence of SIBO in the Korean pediatric population. This study was conducted using a prospective design with adolescent controls. Loose stools were significantly more common, whereas a history of allergies was less common in children with SIBO. Age ≥12 years was an independent factor predicting SIBO in children with FAPDs. This study suggested that SIBO is common in children and adolescents with FAPDs. Among Korean children, especially those aged >12 years and diagnosed with FAPDs, SIBO could be considered the target for treatment to relieve abdominal pain symptoms. In the future, we expect research results on the role of SIBO in various fields, such as clinical symptoms, nutrition, and immune function, under various conditions in which intestinal bacteria may increase in pediatric patients.
Treatment
The principles of treatment are identification and targeting of underlying conditions, symptom control, and nutritional supplements. In addition, selective antibiotic treatment is widely recommended; however, nonantibiotic agents such as prebiotics, probiotics, and prokinetic agents are also being introduced [36,37]. Nutritional supplements should be individualized, and enteral feeding is sometimes necessary. In some patients with diseases such as short-bowel syndrome, a lactose-exclusion diet should be provided, monosaccharide intake reduced, and energy support obtained from fat. In this review, antibiotic treatments are briefly explained.
Antibiotics
Antibiotics, including rifaximin, metronidazole, neomycin, norfloxacin, amoxicillin, and tetracycline, are used to treat SIBO. However, the long-term use of broad-spectrum antibiotics may be inappropriate because of the risk of antibiotic resistance and imbalanced intestinal microbiota. Diarrhea or Clostridium difficile infection can also occur with long-term antibiotic treatment. Rifaximin, a nonabsorbable antibiotic, has been widely used to eradicate SIBO [11,20,21], and it is effective against both gram-positive and -negative strains and aerobic and anaerobic bacteria. Rifamixin for 7–10 days is usually recommended depending on the patient’s condition, although its periodic use is possible. For the treatment of SIBO, high doses (1,200–1,600 mg/day) are more effective than standard doses (600–800 mg/day). Rifaximin not only improves most symptoms but can sterilize up to 80% of overgrown bacteria.
Antibiotic treatment in children
A few studies of antibiotic treatment of pediatric populations have been published, but they have several limitations, including a small number of patients, a lack of controls or specific disease groups, chronic abdominal pain, IBS, and malnutrition. Pimentel et al. [38,39] confirmed that all patients who showed improvement on an H2 breath test experienced overall improvement in IBS symptoms after rifaximin treatment. Among patients whose results did not improve on the breath test, the overall symptoms improved in 32% of the rifaximin treatment group versus 10% of the placebo group. Rifaximin can also be used for a long period of time in patients with IBS and SIBO. However, there are reports that abdominal distention and overall symptoms improve after treatment, whereas diarrhea symptoms do not.
A placebo-controlled randomized controlled trial by Collins and Lin [40] of 75 children with functional abdominal pain found no significant differences in symptom improvement in the rifaximin group (550 mg 3 times a day for 10 days). However, only 20% of the children achieved normalized lactulose breath test profiles after treatment. A subsequent open-label study [21] showed that rifaximin 600 mg/day for 7 days was effective in improving symptoms and treating SIBO in 50 children with IBS. The authors reported significant improvement in IBS symptoms after normalizing the lactulose breath test results (64% of the studied population). The authors attributed the discrepancy in these results to the different criteria used to define positivity on the lactulose breath test and variation in mean subject age and study population composition, which included children with dyspepsia and functional abdominal pain unlike the study by Collins and Lin [40] One study [41] of 20 SIBO patients treated with trimethoprim-sulfamethoxazole and metronidazole for 14 days demonstrated that symptom relief was associated with decreased production of H2 but not CH4.
Overall, these studies have shown mixed results regarding the effects of antibiotics. There are few data on the effect of rifaximin in pediatric patients with IBS symptoms or SIBO; therefore, further investigations are required.
Further investigations
Pediatric SIBO shows wide symptom and severity spectra ranging from IBS-like symptoms to malabsorption or malnutrition. Disturbances in intestinal motility or altered intestinal anatomy are predisposing factors. Treatment principles include managing predisposing conditions, nutritional support, symptom control, and antibiotic treatment. Much research data exist on the diagnosis and treatment of SIBO in adults; however, welldesigned research on children remains lacking. Studies in children are limited by small groups of specific populations, such as those with chronic abdominal pain, disease conditions, or malnutrition. Diagnostic methods and cutoff values require optimization. Further prospective studies are needed of antibiotics that have been proven effective in adults, recurrence rates in pediatric patients, factors that predict treatment failure or recurrence, treatment effects of probiotics, and studies of gut dysbiosis in children.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.