Association between feeding intolerance and intestinal dysbiosis in very premature infants
Article information
To the editor
Premature birth rate in Indonesia is approximately 15% per year and ranks fifth in the world [1]. As survival of premature infants’ increase in recent years, the focus of neonatal care is shifting to feeding optimization. The ability to tolerate enteral nutrition among premature infants is highly dependent on the maturity phase of the gastrointestinal tract, which is associated with motility, digestive enzymes, hormone response, bacterial colonization, and local immune system [2]. Difficulty in ingestion or digestion of nutrition is known as feeding intolerance (FI) [3].
Gut microbiota role in premature infants is widely discussed and considered in recent years [4,5]. A disruption to microbiota homeostasis, called dysbiosis, had been hypothesize as a contributing factors to FI [6]. It is closely related to the growth of pathogenic bacteria, virulence factors, deviation of metabolic function, and changes in the inflammatory process. To understand this phenomenon, we conduct a study to determine whether dysbiosis in premature infants will lead to FI.
This prospective cohort study was conducted at the Neonatology Unit of Cipto Mangunkusumo Hospital (CMH), Jakarta, from November 2019 to January 2021. The inclusion criteria were as follows: very premature infants (gestational age <32 weeks and/or birth weight <1,500 g), admitted in neonatology intensive care unit, and parents’ approval proven by written informed consent. Exclusion criteria were newborn with lethal congenital abnormalities, mothers with incomplete data, and infant who were given probiotics. Potential confounders are respiratory/ventilatory support, necrotizing enterocolitis (NEC), patent ductus arteriosus (PDA), intraventricular hemorrhage (IVH), and type of enteral feeding nutrition. We followed subjects until either discharged or dead.
Our definition of FI was derived from Ahammad [7]; infants who met the following conditions were considered to have FI: a gastric residual volume of more than 50% of the previous feeding volume, and abdominal distension and/or vomiting observed for 3 consecutive days. Our definition of dysbiosis was modified from Peterson [8]; a ratio of commensal microbiota to pathogenic microbiota <1 indicated dysbiosis. In this study, we considered Bifidobacterium sp. and Lactobacillus sp. as commensal bacteria, while Enterococcus sp. and Clostridium sp. as pathogenic bacteria. Fecal microbiota examination was conducted using quantitative real-time polymerase chain reaction (qPCR) method. All samples were examined by certified laboratory officer.
Multiple logistic regression was conducted for multivariate analyses to adjust potential confounding variables. Effect size was estimated using odds ratio (OR) and 95% confidence interval. A P value of ≤0.05 was considered statistically significant. This study was approved by the Health Research Ethics Committee of the Faculty of Medicine Universitas Indonesia-CMH.
A total of 43 infants were analyzed. Thirty-five of the 43 infants (81.3%) had FI. The median time to reach full feeding was 13 days (range, 4–81 days). No difference in the incidence of FI between the method of delivery and use of maternal antibiotics.
The quantities of bacterial DNA obtained from the stool samples are: all bacteria, 1,421.29 (1.20–627,392.78); Lactobacillus sp., 45 (0.11–7,090.76); Enterococcus sp., 22.58 (3.56–4,159.6); and Clostridium sp. 87.89 (45.71–96.50). Due to no Bifidobacterium sp. and only a small number of Clostridium sp. were detected, we define dysbiosis as ratio of Lactobacillus sp.to Enterococcus sp. <1. We found that 28 out of 43 infants (65.1%) had dysbiosis. Overall, distribution of Lactobacillus sp. and Enterococcus sp. did not differ between the 2 groups (Fig. 1).
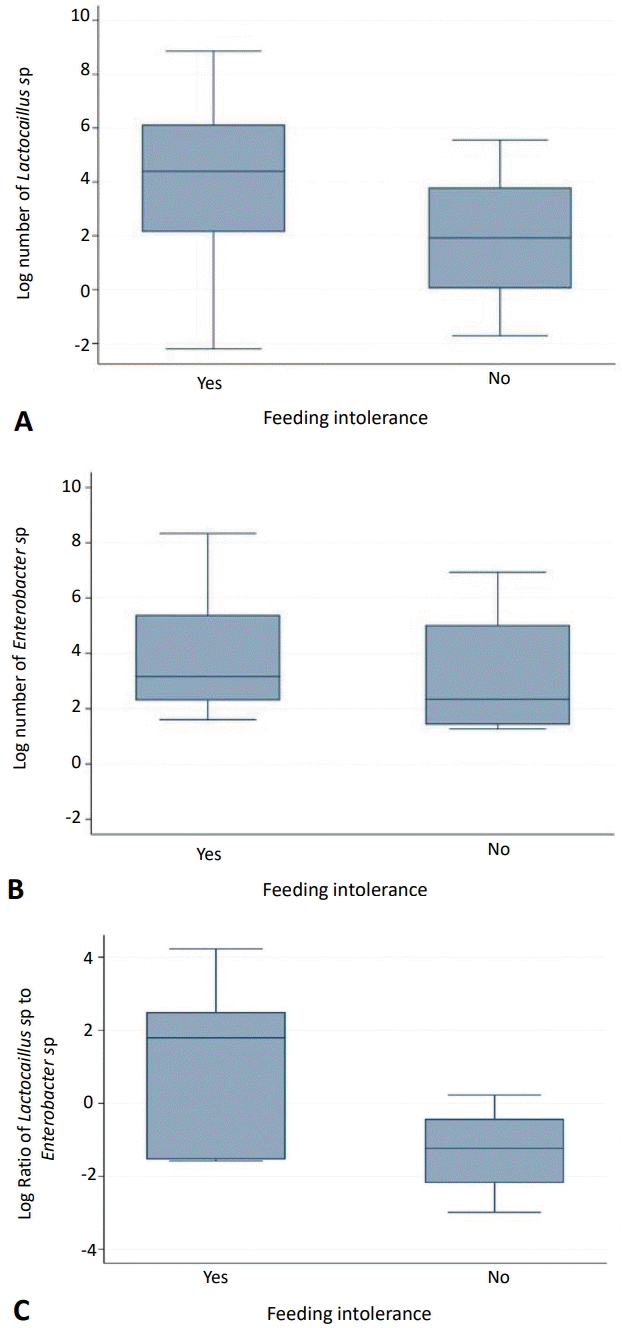
Distribution of gut microbiome between study groups specifically for Lactobacillus sp. (A), Enterococcus sp. (B), and ratio of Lactobacillus sp. to Enterococcus sp. (C). The data are presented as logarithms for the intergroup comparison.
In multivariate analyses (Table 1), dysbiosis did not show any association to FI (OR, 3.26; 95% confidence interval [CI], 0.66–15.98) after adjusted by confounding factors (maternal antibiotic status, PDA, IVH, and NEC). We also found no association between Lactobacillus sp. and Enterococcus sp. composition and mode of delivery, maternal antibiotic use, and type of enteral feeding nutrition.
This is the first study among Indonesian premature infant which evaluate the association between dysbiosis and FI. Infants with FI had slightly lower Lactobacillus sp. and slightly higher Enterococcus sp. although statistically not significant. Otherwise, we could not demonstrate any association between dysbiosis and FI. This finding is valuable in a search of evidence that dysbiosis impact FI among premature infants.
In very premature infants, Enterobacteriaceae and Clostrideaceae are two of the earliest bacteria that colonize the gut, while Bifidobacteriaceae and Lactobacillaceae are characteristically delayed [9]. In the first month of life, low proportions of Bifidobacteriaceae and high proportions of Enterobacteriaceae and Clostridiaceae can lead to dysbiosis. Although no association found between FI and dysbiosis in this study, interestingly, a quantitative bacteria examination showed a slightly lower Lactobacillus sp. and slightly higher Enterococcus sp. among infants with FI [9].
We did not find any difference in the ratio of Lactobacillus sp. to Enterococcus sp. between infants with and without FI. Wen et al. [10] found that the composition of the community microbiota in premature infants diagnosed with FI was significantly different from that in premature infants without FI. Enterococcus sp. significantly increased in healthy premature infants compared to infants with FI. This indicates that Enterococcus sp. might act to maintain intestinal homeostasis in humans [10]. In this study, among infants experiencing dysbiosis, 90% were born through cesarean section and 62% of them received antibiotics. We assumed that these factors would influence their microbiota composition.
We found similar ratio of Lactobacillus sp. to Enterococcus sp. between delivery methods and maternal antibiotic use. Underwood et al. [9] found that the method of delivery and antibiotic use were associated with the composition of the gastrointestinal microbiota. The pattern of microbiota colonization in the cesarean delivery method is dominated by Staphylococcaceae, while that in infants who consumed breast milk was dominated by Bifidobacterium; the bacterial composition was more varied in infants who were given formula milk (Staphylococcaceae, Clostridiaceae, Enterococcaceae, Bifidobacterium, and Bacteroidaceae) [5]. The lack of different microbial pattern in our study was possibly due to the low sample size, technique used to identify microbiota (16S qPCR), and single collection time.
FI and dysbiosis are prevalent in premature infants; however, no association between dysbiosis and FI. Further studies are necessary to better explain the etiology of FI in premature infants.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by Kementerian Riset, Teknologi, dan Pendidikan Tinggi Direktorat Riset dan Pengabdian Kepada Masyarakat through Doctoral Disertation Research Grant (NKB-389/UN2.RST/HKP.05.00/2020).

