Risk factors of prolonged diarrhea in children under 2 years old
Article information
Abstract
Background
Prolonged diarrhea, defined as diarrhea lasting longer than 7 days, is known to negatively impact children's growth and development. However, studies of the risk factors of prolonged diarrhea remain limited.
Purpose
This study aimed to analyze the risk factors for prolonged diarrhea in children under 2 years of age with acute diarrhea.
Methods
This 1-year nested case-control study was conducted at Fatmawati General Hospital in 2021–2022. Participants were selected using a consecutive sampling method from among children under 2 years of age with acute diarrhea within the previous 2–4 days. Children with diarrhea that lasted 7 days were considered positive for prolonged diarrhea, whereas those with acute diarrhea were considered negative. Children with comorbidities such as malnutrition were excluded. Clinical information including age, breastfeeding history, antibiotic exposure history, and nutritional status was recorded. Complete blood count, blood zinc levels, Rotavirus, adenovirus, and norovirus serology, alpha-1 antitrypsin (AAT) levels, and stool analysis were acquired as laboratory data.
Results
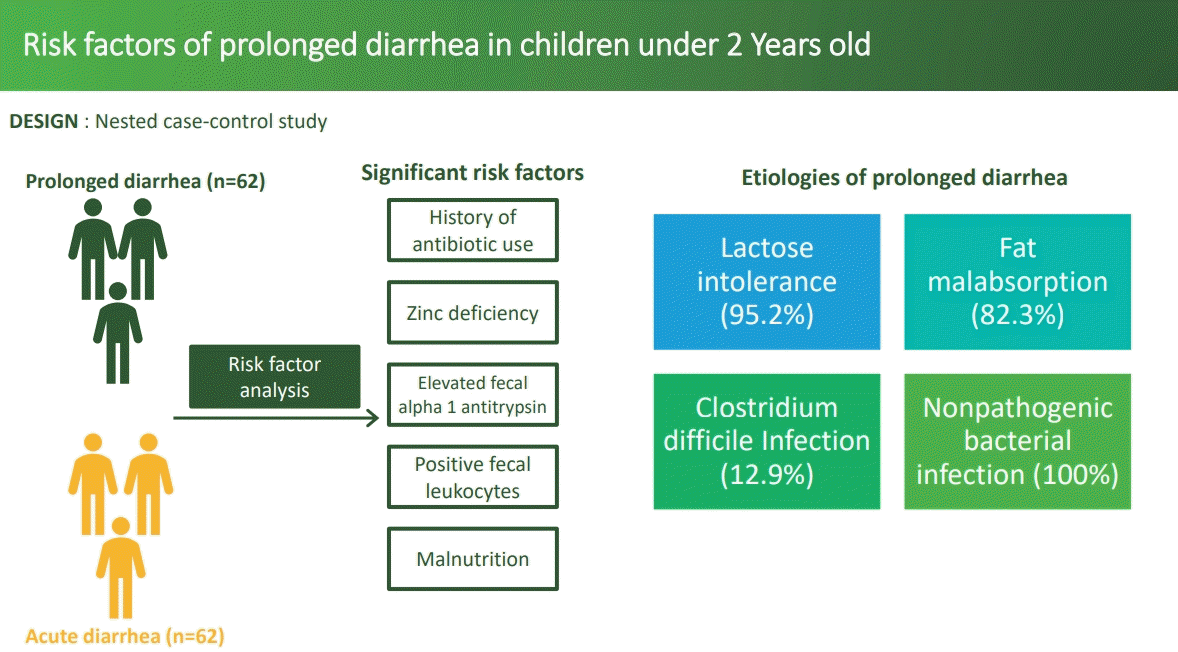
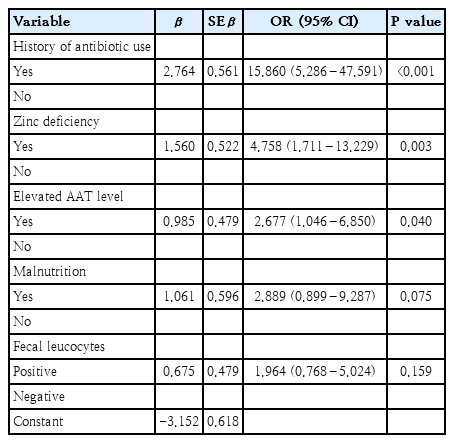
There were 62 subjects in the study and control groups. Overall, the median age was 12 months (6–24 months); most patients were boys. A history of antibiotic use (odds ratio [OR], 15.860; 95% confidence interval [CI], 5.286–47.591; P<0.001), zinc deficiency (OR, 4.758; 95% CI, 1.711–13.229; P=0.003), and elevated fecal AAT levels (OR, 2.677; 95% CI, 1.046–6.850; P=0.040) were the main risk factors for prolonged diarrhea in children under 2 years of age with acute diarrhea.
Conclusion
A history of antibiotic use, zinc deficiency, and elevated fecal AAT levels were the main risk factors for prolonged diarrhea in children under 2 years of age with acute diarrhea. Thorough testing and appropriate antibiotic use are required to prevent prolonged diarrhea in children under 2 years of age.
Key message
Question: What are the risk factors for prolonged diarrhea in children under 2 years old?
Finding: History of antibiotic use, zinc deficiency, and elevated fecal alpha-1 antitrypsin levels were the main risk factors of prolonged diarrhea in children under 2 years old with acute diarrhea.
Meaning: Rational antibiotic usage is necessary as well as thorough testing of serum zinc level and fecal alpha-1 antitrypsin levels.
Graphical abstract
Introduction
Diarrhea is still considered as a global issue due to its tendency to cause significant morbidity and mortality rates among infants and children globally, particularly in developing countries like Indonesia [1,2]. The incidence of diarrhea changes with age. Children under the age of 5 are the most susceptible to diarrhea, accounting for 18% of the fatality rate. The occurrence of diarrhea in children reduces as they grow older [3].
Diarrhea is further classified as acute diarrhea if it lasts for less than 7 days, prolonged diarrhea if it lasts for more than 7 days but less than 14 days, and persistent diarrhea if the episode lasts for 14 days or more [4,5]. In most cases, acute diarrhea will resolve in less than 7 days. However, if the diarrhea lasts longer than 7 days (prolonged diarrhea), then the risk of it to evolve into persistent diarrhea increases [6,7]. Several risk factors such as the use of antibiotics, presence of blood and mucus as well as malnutrition are known to have increased the risk of persistent diarrhea in children. However, study regarding these risk factors for prolonged diarrhea is still limited [5]. Moore et al. [7] reported that children who suffered from prolonged diarrhea had their weightand height-for-age significantly decreased. Furthermore, studies on prolonged diarrhea in children under 2 years old are lacking. Thus, study regarding the risk factors of prolonged diarrhea is crucial to help prevent this debilitating disease. Identifying highrisk children accurately allows clinicians to decide when further advanced tests should be undertaken in the case of acute diarrhea. Lastly, study regarding prolonged diarrhea greatly enhance our understanding of its evolution into persistent diarrhea and may provide hints of potential strategies for its control. Thus, this study aimed to identify the risk factors of prolonged diarrhea in children under 2 years old with acute diarrhea.
Methods
1. Study design and population
This nested case-control study was conducted at Fatmawati General Hospital in 2021–2022 for 1 year. The participants were children under 2 years old with acute diarrhea within the previous 2–4 days in pediatric ward and were selected by consecutive sampling method. Children who had diarrhea for more than 7 days were considered as positive cases of prolonged diarrhea. Meanwhile, children with acute diarrhea (diarrhea that lasted less than 7 days) were considered as negative cases. The exclusion criteria were children on immunosuppressive medication, children with human immunodeficiency virus disease, metabolic disease, and malignant disease, children with dysentery (bloody diarrhea), children with hospital-acquired diarrhea, children with comorbidities, and those with a history of gastrointestinal surgery.
2. Study definitions
Prolonged diarrhea was defined as diarrhea persisted for ≥7 days. Children were considered as exclusively breastfed if they were breastfed for at least 6 months. According to World Health Organization Growth Chart 2006, overweight was defined as a weight-for-height z score of >2 standard deviation (SD), undernutrition was defined as a weight-for-height z score of <-2 SD, and malnutrition was defined as a weight-for-height z score of <-3 SD. Zinc deficiency was defined when serum zinc level was lower than 65 µg/dL or 9.9 µmol/dL. Iron-deficiency anemia was defined when hemoglobin level was lower than 11 g/dL and mean corpuscular volume<70 fL with microcytic hypochromic erythrocyte and normal red cells distribution width. Rotavirus, adenovirus, and norovirus were detected by using stool rapid test CerTest (CerTest Biotec, Zaragoza, Spain). Antibiotic exposure was considered positive if the patients were exposed to antibiotics within 2 months of the onset of acute diarrhea. Fecal leucocyte was considered as positive if there were ≥10 leucocytes per high power field. Fecal alpha 1-antitrypsin (AAT) level was measured by using standard enzyme-linked immunosorbent assay method. Elevated fecal AAT was defined as AAT level of ≥ 50.8 mg/dL.
3. Study outcomes
History taking, physical examination, and supporting laboratory tests were collected to identify the risk factors of prolonged diarrhea in children under 2 years old. History taking was performed to obtain information regarding the duration of acute diarrhea, age, history of breastfeeding, and history of antibiotic exposure. Physical examination was conducted to determine the nutritional status of the children, as well as to exclude any comorbidities. The laboratory tests were complete blood count, blood zinc levels, Rotavirus, adenovirus, and norovirus serology, AAT level, and stool analysis. Children whose diarrhea persisted for more than 7 days were continued to be followed up until recovery while laboratory works for lactose intolerance, fat malabsorption, Clostridium difficile toxin, and fecal culture were performed. Fat malabsorption was measured using a steatocrit test, which was also done after 7 days in the study group. Treatment of acute diarrhea followed the standard protocol published by the Ministry of Health Republic of Indonesia [8].
4. Statistical analysis
Statistical data was collected and analyzed using IBM SPSS Statistics ver. 25.0 (IBM Co., Armonk, NY, USA). Bivariate analysis was evaluated using chi-square test or Fischer test as appropriate. Multivariate analysis was performed using logistic regression analysis with backward stepwise method. Data were presented as odd ratio with 95% confidence interval. A P value of <0.05 was considered statistically significant.
5. Ethical considerations
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethical Committee of FKUI-RSCM (number 209/UN2.F1/ETIK/2015). Parents from each patient provided consent for inclusion in this study and signed the written consent form.
Results
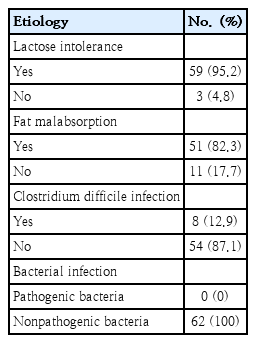
There were 152 children eligible for this study, which consisted of 62 subjects with diarrhea that lasted ≥7 days (prolonged diarrhea), while 90 subjects who recovered from acute diarrhea in <7 days were chosen with simple randomization to include 62 subjects as a control group. The overall median age was 12 months old (6–23 months) in the positive group and 12.5 months old (6–24 months) in the control group. Boys dominated both groups in terms of proportion. Meanwhile, the overall median duration of hospitalization was 4 days (2–14 days). The basic clinical characteristics and laboratory of the subjects are presented in Table 1. There was no difference in basic characteristics between prolonged diarrhea group and control group. The etiologies of prolonged diarrhea are presented in Table 2. Fecal culture revealed nonpathogenic bacteria such as nonpathogenic Escherichia coli, Klebsiella oxytoca, Pseudomonas aeroginosa, and Raoultella ornithinolytica in all 62 patients.
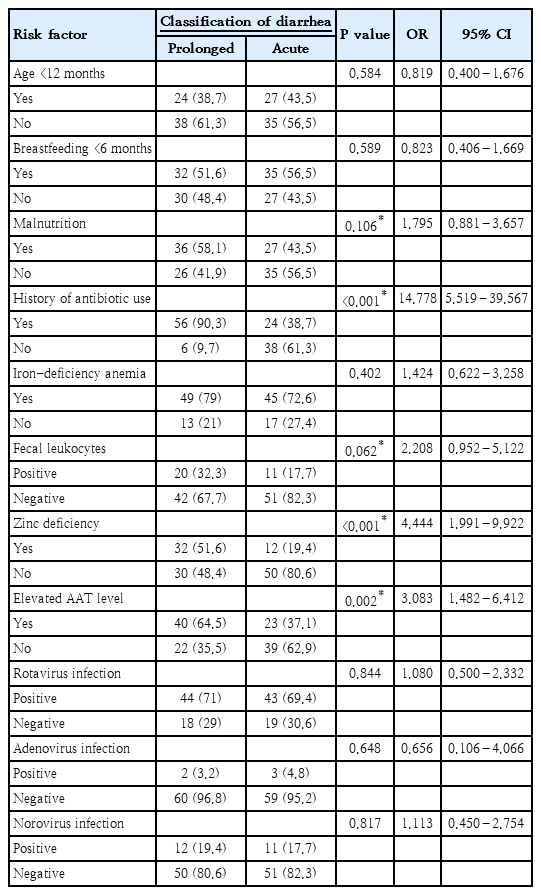
Based on bivariate analysis, the significant risk factors associated with prolonged diarrhea were the history of antibiotic use, zinc deficiency, elevated AAT levels, fecal leukocytes, and malnutrition. The result of bivariate analysis of all risk factors associated with prolonged diarrhea were presented in Table 3. Risk factors with P value of <0.25 were then analyzed further with multivariate analysis of logistic regression and the results found that the history of antibiotic use, zinc deficiency, and elevated fecal AAT levels were the significant risk factors of prolonged diarrhea in children under 2 years old with acute diarrhea (Table 4). The logistic regression model improved significantly compared to constant-only model, X2(5)=58.551 (P<0.001). Furthermore, Hosmer-Lemeshow test was not statistically significant (P>0.05), which indicated that the actual number of cases were not significantly different from the cases predicted by the model and had a satisfactory goodness of fit. This model explained 50.2% (Nagelkerke R2) of the variance in prolonged diarrhea, and correctly predicted 82.3% of cases.
Discussion
In this study, various risk factors of prolonged diarrhea in children under 2 years old with acute diarrhea were evaluated. The results of this study demonstrated that the significant risk factors associated with prolonged diarrhea were positive history of antibiotic use, zinc deficiency, elevated AAT levels, positive fecal leukocytes and malnutrition.
History of antibiotic use in this study was observed in 64.5% of all patients with 90.3% of patients with prolonged diarrhea reported the history compared to only 38.7% in the control group. The incidence of antibiotic-associated diarrhea (AAD) caused by C. difficile toxin among our prolonged diarrhea patients was 12.9% in this study. This result is similar to that reported by Turck et al. [9] who observed the incidence of AAD of 11%, while study from Vanderhoof et al. [10] observed a higher incidence rate of 26%. Study in Thailand had a lower incidence rate of 6.2% [11]. This study further proved that the history of antibiotic use was statistically significant to be considered as a risk factor for the occurrence of prolonged diarrhea in children under 2 years old with acute diarrhea. Several class of antibiotics such as cephalosporins, penicillin and azithromycin are the most readily to induce AAD, with even higher risk if those antibiotics are combined [12]. Administration of antibiotics disrupts the intestinal microflora by killing the antibiotic-sensitive beneficial bacteria while drug-resistant pathogenic bacteria multiply. Decline of beneficial bacteria in the colon reduces the amount of short chain fatty acid especially butyrate, a byproduct of carbohydrate metabolism which is a primary source of energy for colonic mucosal layer. Consequently, osmotic diarrhea occurs due to high quantity of carbohydrate in the colon as well as impaired mucosal layer [13]. Furthermore, exposure to antibiotics particularly in early life, is notorious to alter the immune system by disrupting the balance between T-helper 1 and T-helper 2 [14].
The overall prevalence of zinc deficiency in this study was 35.5%, and more commonly found in the prolonged diarrhea group (51.6%) compared to the acute diarrhea group (19.4%). The prevalence of zinc deficiency in this study is comparable to other studies. Study conducted in South and Southeast Asia showed zinc deficiency rates ranging from 34%–73%, and study in West Java, Central Java, and Lombok had a prevalence rate of 6%–39%. The prevalence of zinc deficiency in prolonged diarrhea group in this study was higher than that reported by Bitarakwate et al. [15] who observed the prevalence of 47.9% in children with persistent diarrhea in Uganda. Zinc is an essential micronutrient that plays an important role in regulating the immune system especially in children. Both innate and adaptive immune system requires the presence of this element to effectively clear out infections within the body. Moreover, zinc also takes part in immune cells development as well as maturation of T cells [16]. Therefore, deficiency of this element may result in prolonged diarrhea due to ineffectiveness of the immune system to eradicate the infection associated with diarrhea. The results of this study also indicated the importance of zinc supplementation in patients with acute diarrhea. Furthermore, Hidayat et al. [17] proved that zinc supplementation in children with acute diarrhea will shorten the period of diarrhea significantly.
Positive fecal leukocytes were found in 25% of our study population with higher positive rate was found in the prolonged diarrhea group (32.2%) compared to the acute diarrhea group (17.7%). The percentage of positive fecal leukocytes in this study was higher than that reported by Mercado et al. [18] who observed 11.8% of positive leukocytes among children with diarrhea in general. Higher percentage of positive fecal leukocytes was reported by Moore et al. [7] who obtained 50.8% of positive stool leukocytes in children with prolonged diarrhea compared to only 32.2% in our study. This disparity was mainly due to the different etiology in both studies as Shigella and Cryptosporidium were the main cause of prolonged diarrhea in study by Moore et al. [7], while our study reported that Rotavirus as the main cause. Furthermore, positive fecal leukocyte is mainly considered as a marker of inflammatory diarrhea associated with invasive pathogens such as Salmonella, Shigella, Campylobacter, Yersinia, and entero-invasive E. coli [18]. Nevertheless, sometimes noninvasive pathogens can elicit a mild inflammatory response as a result of the interaction of the pathogen with the host′s enteric cells. The World Health Organization recommends that the positive fecal leukocyte count of ≥10 per high power field is considered a marker of pathogenic bacterial infection, and thus, empiric antibiotic treatment is recommended according to epidemiological data especially in areas where stool culture is not readily available [19]. Therefore, delay in providing adequate antimicrobial treatment may lead to prolonged diarrhea in children with acute diarrhea associated with invasive bacterial pathogens.
Elevated fecal AAT was observed in 64.5% of children with prolonged diarrhea compared to 37.1% of those with acute diarrhea. Fecal alpha-1 antitrypsin is an indicator of protein losing enteropathy, a condition in which there is an excessive loss of protein from the gastrointestinal tract due to various etiologies. Three main groups of disorders that cause protein loss in stool are erosive gastrointestinal disorders, nonerosive gastrointestinal disorders and disorders causing increased interstitial pressure. However, elevated fecal AAT also occurs in diarrhea caused by Rotavirus, adenovirus, Shigella, enterotoxigenic E. coli, and Salmonella as these pathogens cause disruption in the tight junction of the gastrointestinal tract and consequently leads to protein leakage. Therefore, elevated fecal AAT level in a case of diarrhea is an excellent marker of protein loss as well as a marker for impaired tight junction along the gastrointestinal tracts, that warrants further investigation to determine the underlying etiology in order to prevent the occurrence of prolonged diarrhea. Higher proportion of elevated fecal AAT was observed in prolonged diarrhea group compared to acute diarrhea group in our study, which indicated that prolonged diarrhea is more likely to occur when there is a disturbance in tight junction as well as protein loss.
In this study, the prevalence of malnutrition among our study population was 50.8% with higher number was observed in those with prolonged diarrhea (58.1%) compared to those with acute diarrhea (43.5%). Based on the result of our study, children with malnutrition were 2.8 times more likely to developed into prolonged diarrhea. Similar finding was also reported by Putra et al.[20] as well as Moore et al. [7]. The relationship between malnutrition and diarrhea is bidirectional in which malnutrition impairs the mucosal barrier of the gastrointestinal tract and disrupts the immune system which will eventually prolong the duration of the immune system to effectively clear the infection associated with diarrhea. Furthermore, malnutrition is also associated with micronutrient deficiency such as zinc, vitamin A and iron, which contributes to the delay of gastrointestinal tissue recovery and thus causing prolonged diarrhea [21]. Malnutrition is still one of the leading causes of increasing morbidity and mortality in children in all parts of the world especially in developing countries such as Indonesia. Higher risk of diarrhea in children is often associated with the incidence of malnutrition, whereas if children with good nutritional status experience diarrhea, their nutritional status may decline as a consequence. Moreover, malnutrition is also associated with high mortality in children with diarrhea [22,23].
There are some novel aspects in this study. This is the first study to completely evaluate the numerous risk factors for prolonged diarrhea in a single study, including additional risk factors such as zinc deficiency, adenovirus infection, and norovirus. This study also identified several etiological factors of prolonged diarrhea, including lactose intolerance, fat malabsorption, and C. difficile infection. Overall, this study found that history of antibiotic use, zinc deficiency, and elevated fecal AAT levels were the major risk factors of prolonged diarrhea in children under 2 years old with acute diarrhea. In order to prevent prolonged diarrhea in children under 2 years old, thorough testing and appropriate antibiotic use are necessary.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
AF, PG; Formal Analysis: DR, IST, SB; Investigation: DR; Methodology: JP, IST, SB; Project Administration: DR; Writing-Original Draft: DR, AF, IST, SB, JP, PG; Writing-Review Editing: DR, AF, IST, SB, JP, PG
Acknowledgements
We would like to acknowledge all parents who had provided their consent for this study.