Oral administration of bone marrow-derived mesenchymal stem cells attenuates intestinal injury in necrotizing enterocolitis
Article information
Abstract
Background
Necrotizing enterocolitis (NEC) is a major cause of morbidity in premature infants. However, effective treatment options for NEC are currently lacking.
Purpose
This study aimed to determine the optimal dose of intraperitoneally administered bone marrow-derived mesenchymal stem cells (BM-MSCs) and investigate the therapeutic potential of orally administered BM-MSCs in NEC.
Methods
Neonatal mice were fed maternal breast milk for the first 2 days of life. On day 3, the neonatal mice were randomly divided into control, negative control, and BM-MSC-treated groups. Lipopolysaccharide (LPS) was administered for 3 days, and cold stress (4°C, 10 minutes) was applied 3 times a day to induce NEC. High-dose (1×106 cells) or low-dose (1×105 cells) BM-MSCs were administered intraperitoneally 1 or 3 times between days 6 and 8 to treat the NEC. The orally administered group received a low dose of BM-MSCs on day 6. Furthermore, except for the control group, intraepithelial cells (IECs) of the small intestine of neonatal mice were treated with LPS and exposed to 5% O2/95% N2 hypoxic stress for 2 hours. Thereafter, each was treated with BM-MSCs.
Results
Tissue injury, apoptosis, and inflammatory marker levels were significantly reduced after BM-MSC administration. Oral administration was as effective as intraperitoneal administration, even at a low dose (1×105 cells) of BM-MSCs. The efficacy of high (1×106 cells) or multiple divided doses of BM-MSCs did not differ from that of low-dose treatment. Significantly improved wound healing was observed after BM-MSC administration to injured IECs.
Conclusion
The oral administration of BM-MSCs is a promising treatment option for NEC in infants. Further human studies of BM-MSCs are necessary to determine the optimal dose required to achieve safe and effective outcomes.
Key message
Question: What is the optimal dose of bone marrow-derived mesenchymal stem cells (BM-MSCs) for treating necrotizing enterocolitis (NEC), and is orally administered BM-MSC effective?
Finding: High (1×106 cells) or multiple BM-MSC doses showed similar effects as low (1×105 cells) doses of intraperitoneally administered BM-MSCs. Furthermore, orally administered BM-MSCs were as effective as intraperitoneally administered BM-MSCs.
Meaning: Orally administered low-dose BM-MSCs are a potential treatment for NEC.
Graphical abstract. The review includes the applications of new technologies for medical services targeting pediatric patients and training methods for medical professionals. There are programs designed to reduce children’s pain, anxiety, and fear during in-hospital treatment (left), and medical staff’s education for child patients (right). BM-MSCs, bone marrow-derived mesenchymal stem cells.
Introduction
Necrotizing enterocolitis (NEC) is a fatal gastrointestinal disorder affecting 7%–10% of premature infants [1]. It manifests as sudden inflammation and necrosis of the intestine, leading to systemic sepsis [2]. Current treatment options are limited to supportive care such as fasting and systemic antibiotic use. Infants who survive NEC often experience long-term complications [3].
NEC has a multifactorial etiology with several risk factors associated with its development. Premature birth is the most common risk factor, accounting for 90% of all cases [4]. Other known risk factors include excessive use of antibiotics [5], administration of acid-suppressing H2 blockers [6], hypoxia episodes [7], maternal chorioamnionitis [8], and formula feeding [9]. Based on these known risk factors, various experimental animal NEC models have been developed using cold stress; hypoxic stress; and administration of formula feed, endotoxins, or lipopolysaccharides (LPS) [10-12]. These models have demonstrated significant apoptosis of enterocytes in the apical villi, a phenomenon is commonly observed in patients with NEC.
Recently, cell-based therapies using bone marrow-derived mesenchymal stem cells (BM-MSCs) have gained attention because of their self-renewal, multipotent properties, and immunomodulatory effects by regulating immune cells within inflamed and damaged tissues [13,14]. Bone marrow-derived mesenchymal stem cells are currently used to treat various chronic inflammatory or autoimmune conditions, including inflammatory bowel disease [15], graft-versus-host disease [16], and acute pancreatitis [17]. Furthermore, BM-MSCs are recognized as candidates for treating NEC [18-20].
Previous study has demonstrated that the intraperitoneal (IP) or intravenous (IV) administration of BM-MSCs decreases intestinal permeability, improves gut barrier function [18], and reduces NEC incidence [19]. However, there is a lack of studies conducted to determine the optimal dose of BM-MSCs to treat NEC. In addition, the oral administration of drugs is clinically preferred in premature infants to minimize invasive procedures and potential complications. Currently, there is a lack of comprehensive research on the effectiveness of oral administration of BM-MSCs in animal models. Therefore, in this study, we aimed to determine the optimal dose of IP BM-MSCs and investigate the therapeutic potential of orally administered BM-MSCs for NEC.
Methods
1. Preparation of BM-MSCs
Allogeneic BM-MSCs that can differentiate into 3 mesenchymal lineages (osteoblasts, adipocytes, and chondrocytes) and express specific surface markers of general MSCs in a particular pattern were provided by SCM Life Sciences Co., Ltd. (Incheon, Korea). Cells at passage 8 were used in this study.
2. In vivo study
1) Animal model study
All animal experiments were approved by the University Institutional Animal Care and Use Committee (INHA 190517-647). Seven pregnant ICR mice were obtained from Orient Bio (Seongnam, Korea). On day 18 of gestation, 118 neonatal mice were delivered spontaneously. The neonatal mice were fed maternal breast milk for 2 days after birth. On day 3, these neonatal mice were randomly divided into 7 groups (group 1, n=13; group 2, n=22; group 3, n=17; group 4, n=14; group 5, n=17; group 6, n=17; and group 7, n=18).
LPS (Sigma-Aldrich Co., St. Louis, MO, USA; 5 mg/kg/day) was administered intragastrically using a 26-Fr angiocatheter for 3 days, whereas cold stress (4°C, 10 minutes) was applied thrice a day to neonatal mice belonging to groups 2–7 to induce NEC. Mice belonging to all study groups, except the control group (group 1), were IP administered (group 7 orally) the following treatments (Fig. 1): Group 2 (NEC+phosphate-buffered saline [PBS]), 0.1 mL PBS IP on day 6; Group 3 (NEC+low dose [LD]-MSCs#1), 1×105 BM-MSCs suspended in 0.1 mL on day 6; Group 4 (NEC+LD-MSCs#3), 3.3×104 BM-MSCs suspended in 0.1 mL each day from day 6 to 8; Group 5 (NEC+high dose [HD]-MSCs#1), 1×106 BM-MSCs suspended in 0.1 mL on day 6; Group 6 (NEC+HD-MSCs#3), 3.3×105 BM-MSCs suspended in 0.1 mL each day from day 6 to 8; Group 7 (NEC+LD-MSCs#1), 1×105 BM-MSCs on day 6 orally using a 26-Fr angiocatheter.

Experimental design of in vivo study. Mice were delivered on day 18 (E18) of gestation. The neonatal mice were subjected to LPS treatment and hypothermia for 3 days to induce NEC (groups 2–7). On day, groups 3–7 were administered BM-MSCs. Mice of all groups were sacrificed on day 9 of life. BM-MSC, bone marrow-derived mesenchymal stem cell; NEC, necrotizing enterocolitis; PBS, phosphate-buffered saline; LD-MSC, low-dose mesenchymal stem cell; BM-MSCs, bone marrow-derived mesenchymal stem cells; LPS, lipopolysacc haride.
For administration, BM-MSCs were suspended in 0.1 mL of Dulbecco's modified Eagle’s medium (DMEM) low glucose supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA). All neonatal mice were sacrificed at the same time on day 9, and the entire small intestines were harvested for analysis.
2) Histological examination
The prepared tissue samples were fixed in 10% formalin and embedded in Paraffin. The Paraffin-embedded sections were stained with hematoxylin and eosin (H&E). Histological examination of the sections was performed by independent observers. Six randomly selected rats per group were included in the analysis, and 5 images per slide were captured using an optical microscope. Following the method described by Lee et al. [21], H&E staining was scored on a scale of 0–4 as follows: 0, intact villi; 1, sloughing of cells at the villous tips; 2, mid-villous damage; 3, loss of villi but readily detectable crypts; and 4, complete absence of epithelial structures and transmural necrosis. To calculate NEC incidence, NEC was determined based on H&E staining scores equal to or higher than 2.
3) Immunohistochemistry
Immunohistochemistry was performed to detect apoptosis using the terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay and identify toll-like receptor 4 (TLR4) expression. The TUNEL assay was conducted using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Millipore Sigma, Burlington, MA, USA) following the manufacturer’s instructions. Apoptosis was scored according to the method described by Jilling et al. [10] as follows: 0, continuous villous epithelium and only solitary TUNEL-positive nuclei; 1, clusters of TUNEL-positive nuclei at the villous tips; 2, apoptotic nuclei covering the villi with complete sparing of the crypts; 3, apoptosis penetrating the crypts; and 4, transmural distribution of TUNEL-positive nuclei. To calculate NEC incidence, NEC was determined based on apoptosis scores equal to or higher than 2.
To determine the expression of TLR4, the tissue sections were incubated with the primary antibody anti-TLR4 (Abcam, Cambridge, UK) overnight at 4°C. The sections were then incubated with goat anti-mouse IgG-HRP (Santa Cruz Biotechnology, Dallas, TX, USA) as the secondary antibody at 25°C for 1 hour in the dark. The histoscore method described by Kauppila et al. [22] was used to combine the intensity and percentage of positive cells. Briefly, staining intensity was scored on a scale of 0–4: 0, negative; 1, weak; 2, moderate; and 3, strong. The extent of staining was assessed as a percentage of positive cells (0%–100%) at 10% intervals. The histoscore was calculated by multiplying the intensity level and percentage of positive cells, resulting in values between 0 and 300. The apoptosis score and TLR4 expression were assessed by 2 independent researchers who were blinded to the data.
3. In vitro study
1) Cell culture and preparation
To obtain intestinal epithelial cells (IECs), the small intestines were harvested from 47 neonatal mice on day 3 after spontaneous delivery from 4 pregnant ICR mice on gestational day 18. The cells were then treated with DMEM supplemented with 1 mM dithiothreitol and 0.5 mM ethylenediaminetetraacetic acid. After incubation at 37°C under 5% CO2 for 30 minutes, the cells were centrifuged at 300×g for 5 minutes at 25°C. The obtained pellet was suspended in DMEM/nutrient mixture F12 (DMEM/F12, Thermo Fisher Scientific) supplemented with 4% FBS (Thermo Fisher Scientific). The cells were passaged when they reached 80%–90% confluence and used at passage 2. Once a monolayer was formed,the cells were serum-starved overnight. The cells were divided into 3 groups and seeded in three 6-well culture plates precoated with collagen. The groups were designated as follows: group 1, control; group 2, NEC+PBS; and group 3, NEC+BM-MSCs. The cells in groups 2 and 3 were treated with LPS (50 μg/mL in 0.5 mL of DMEM/F12) and exposed to hypoxic stress of 5% O2/95% N2 using a multigas incubator (Astec, Seoul, Korea). After 2 hours of exposure, the cells were washed thrice with PBS.
2) Wound healing assay
Alinear scrape wound was created in the IEClayer using a sterile pipette tip (200 μL). Subsequently, 0.5 mL of DMEM/F12 was added to group 1, 0.5 mL DMEM+0.25 μL PBS was added to group 2, and 0.5 mL DMEM+0.25 μL (1.5×105 BM-MSCs) was added to group 3. Inverted phase-contrast light microscopy was used to capture photomicrographs immediately at 0, 4, 10, and 24 hours after wound creation (Supplementary Fig. 1). The experiment was conducted in duplicate, and the wound area in each photomicrograph was measured using ImageJ software version 1.53 (National Institutes of Health, Bethesda, MD, USA).
3)Reverse transcription-quantitative polymerase chain reaction
To the prepared IECs, 0.5 mL of DMEM/F12 (group 1), 0.5 mL of DMEM+0.25 μL PBS (group 2), and 0.5 mL of DMEM+0.25 μL (1.5×105 BM-MSCs) were added. Thereafter, the cells were incubated at 37°C under 5% CO2 for 24 hours. Reverse transcription (RT) was performed using AccuPower RT Premix (Bioneer Corporation, Daejeon, Korea).
The sequences of specific primers used for mouse Il10, Tnfa, and Gapdh were as follows: Il10 [sense, 5-TGGCCC AGAAATCAAGGAGC-3; antisense, 5-TGGCCCAGAAATC AAGGAGC-3], Tnfa [sense, 5-CACAGAAAGCATGATCCG CGACGT-3; antisense, 5-CGGCAGAG AGGAGGTTGACT TTCT-3 ], and Gapdh [sense, 5-TAATGAGCTGGTCATCC GTG-3; antisense, 5-CAGGCTTCCCTGAGTTCATC-3]. Quantitative polymerase chain reaction (qPCR) was performed using the Power SYBR Green Master Mix Kit (Thermo Fisher Scientific). The reaction (with 20-μL mixture) was conducted (95°C for 2 minutes, followed by 40 cycles at 95°C for 15 seconds and 60°C for 60 seconds) in a 96-well plate using the StepOnePlus Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). The fold change in gene expression was calculated using the 2-ΔΔCt method.
4. Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). Data are expressed as percentage (%) or mean±standard error of the mean. Fisher exact test was used to compare the incidence of NEC among the groups in the H&E and TUNEL staining analyses. Kruskal-Wallis and Mann-Whitney U tests were used to compare the TLR4 histological scores among the groups. A 1-way analysis of variance was used to compare gene expression. Alinear mixed model was used to compare wound area. A post hoc analysis was conducted using Bonferroni method. Statistical significance was set at P<0.05.
Results
1. BM-MSCs attenuate intestinal injury in an in vivo model of NEC
1) BM-MSCs ameliorate NEC-associated histological injury in neonatal mice
Histological NEC injury, determined using H&E staining, was scored (Fig. 2A), and the overall NEC incidence (scores 2 or higher) was compared among the groups (Fig. 2B). The incidence of NEC in each group was as follows: group 1 (control), 13.3%; group 2 (PBS after NEC induction), 70%, groups 3–6 (various doses and frequencies of BM-MSC administration after NEC induction), 6.7%, 26.6%, 16.6%, and 0%, respectively; and group 7 (orally administered BM-MSCs after NEC induction), 3.3%. The incidence of NEC was significantly higher in group 2 than in the other groups. However, no significant differences were observed in the incidence of NEC among other treatment groups.
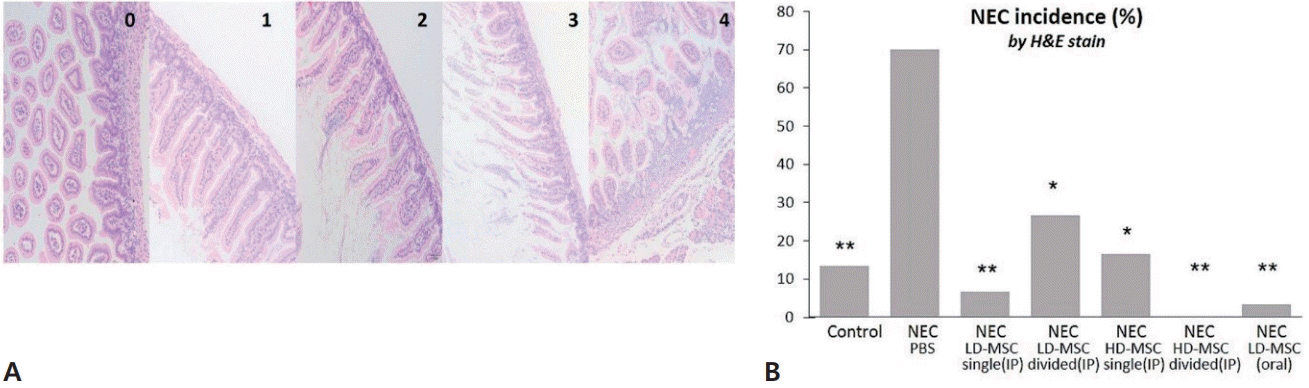
Hematoxylin and eosin (H&E) staining to assess the effect of BM-MSCs on NEC. (A) The images represent injury scores ranging from 0 to 4, and injury scores of 2 or higher were used to determine NEC incidence. (B) The incidence of NEC was calculated for the 7 mouse groups. The data are expressed as percentages and were compared using Fisher exact test with Bonferroni correction. BM-MSC, bone marrow-derived mesenchymal stem cell; NEC, necrotizing enterocolitis; PBS, phosphate-buffered saline; LD-MSC, low-dose mesenchymal stem cell; HD-MSC, high-dose mesenchymal stem cell; IP, intraperitoneal. *P<0.05, **P<0.001, significant compared to group 2 (NEC+PBS).
2) BM-MSCs suppress apoptosis in neonatal mice
Apoptosis was scored using the TUNEL assay (Fig. 3A), and the incidence of NEC (scores 2 or higher) was compared among the groups (Fig. 3B). The incidence of NEC was significantly high in group 2 at 60%; whereas in groups 1 and 3–7,the incidence ofNEC was 20%, 6.7%, 16.7%, 16.7 %, 20%, and 3.3%, respectively. However, no significant differences were observed in the incidence of NEC among the groups. The results indicate that both IP and oral administration of BM-MSCs had similar effects in reducing apoptosis caused by NEC, as observed in the results of H&E staining analysis.
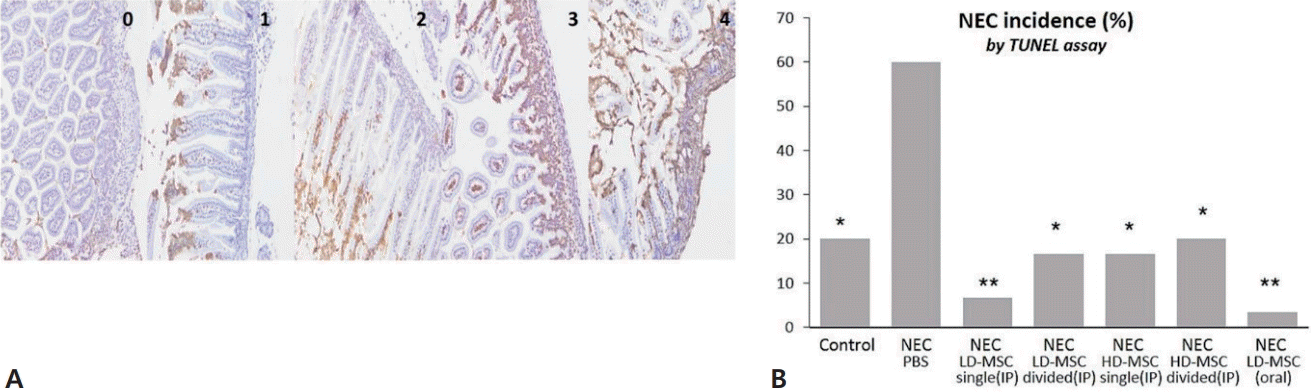
TUNEL staining to assess the effect of BM-MSCs on NEC. (A) The images represent apoptosis scores ranging from 0 to 4, and apoptosis scores of 2 or higher were used to determine NEC incidence. (B) The incidence of NEC was calculated for the 7 mouse groups. The data are expressed as percentages and were compared using Fisher exact test with Bonferroni correction. TUNEL, terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling; BM-MSC, bone marrow-derived mesenchymal stem cell; NEC, necrotizing enterocolitis; PBS, phosphate-buffered saline; LD-MSC, low-dose mesenchymal stem cell; HD-MSC, high-dose mesenchymal stem cell; IP, intraperitoneal. *P<0.05, **P<0.001 significant compared to group 2 (NEC+PBS).
3) BM-MSC administration reduces TLR4 expression in neonatal mice
The immunohistochemical staining intensity (Fig. 4A) and abundance of TLR4 were compared among the study groups (Fig. 4B). The mean TLR4 histological score for group 2 was the highest at 39; whereas, the mean TLR4 histological scores for group 1 and groups 3–7 were 8.7, 10, 9.7, 6.3, 8, and 10, respectively. However, no significant differences were observed in the TLR4 histological scores among the groups. These results, consistent with previous analysis results, indicate that BM-MSC administration inhibits TLR4 expression, regardless of the dose, frequency, or route of administration.

Staining intensity and effect of BM-MSCs on TLR4 expression. (A) The images represent staining intensity scores ranging from 0 to 3. (B) The histological score was calculated by multiplying the intensity level and percentage of positive cells and was compared among the 7 mouse groups. The data are expressed as mean±standard error of the mean and were compared using the Kruskal-Wallis test and Mann-Whitney U test with Bonferroni correction. BM-MSC, bone marrow-derived mesenchymal stem cell; TLR4, toll-like receptor 4; NEC, necrotizing enterocolitis; PBS, phosphate-buffered saline; LD-MSC, low-dose mesenchymal stem cell; HD-MSC, high-dose mesenchymal stem cell; IP, intraperitoneal. *P<0.05, **P<0.001 significant compared to group 2 (NEC+PBS).
The results of the in vivo study suggest that low or high IP doses and single dose or multiple divided IP doses of BM-MSCs aid in the recovery of mice from NEC injury, similar to the effect of oral administration of BM-MSCs.
2. BM-MSCs promote the recovery of intestinal injury in an in vitro model of NEC
1) BM-MSCs foster IEC proliferation after injury
The results of the in vitro would healing assay showed gradual wound healing in all groups over time (Fig. 5A). Significantly improved wound healing was observed in the control(group 1) and group 3 compared with that in group 2 (Fig. 5B). No significant differences were observed between groups 1 and 3. Therefore, the administration of BM-MSCs effectively enhanced wound healing.

Time-dependent healing of scrape wounds in intestinal epithelial cells. (A) Inverted phase-contrast micrographs of scraped wound after 0, 4, 16, and 24 hours. (B) The area of the wound at various time points was calculated in each group and plotted. The data are expressed as mean±standard error of the mean and were compared using the linear mixed model. BM-MSC, bone marrow-derived mesenchymal stem cell; NEC, necrotizing enterocolitis; PBS, phosphate-buffered saline. *P<0.05 significant compared to group 2 (NEC+PBS).
2) BM-MSCs have an immunomodulatory effect on inflammation in an IEC line in vitro
To evaluate the effect of BM-MSCs on the inflammatory response in NEC, the mRNA expression levels of Il10 and Tnfα were assessed in IECs using RT-qPCR. Compared with that in the induced NEC group (group 2), Il10 expression was significantly increased in groups 1 and 3 (Fig. 6A). The mean fold change in Tnfα expression in group 2 was higher than that in groups 1 and 3, although the difference was not significant (Fig. 6B). These findings indicate that MSCs exhibit an anti-inflammatory effect on NEC.
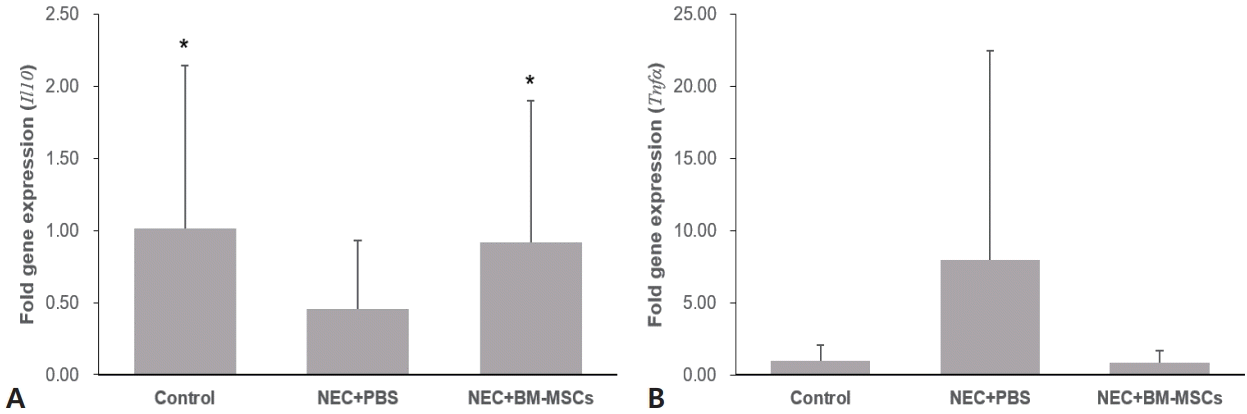
Quantification of Il10 (A) and Tnfa (B) in intestinal epithelial cells using quantitative polymerase chain reaction. The data are expressed as mean±standard error of the mean and were compared using 1-way analysis of variance. NEC, necrotizing enterocolitis; PBS, phosphate-buffered saline; BM-MSC, bone marrow-derived mesenchymal stem cell.
Discussion
In this study, we aimed to determine the therapeutic potential of BM-MSCs for NEC. Additionally, we conducted experiments to adjust the appropriate dose and frequency of BM-MSC administration via the IP route; furthermore, BM-MSCs were orally administered to evaluate their effectiveness. We demonstrated that the administration of BM-MSCs reduced tissue injury, apoptosis, and inflammatory marker level; improved wound healing; and exhibited anti-inflammatory effects. Furthermore, the administration of BM-MSCs at a low dose (1×105 cells) showed a similar improvement in NEC as higher (1×106 cells) or multiple dose administration of BM-MSCs. Moreover, the oral administration of BM-MSCs showed effects comparable to those ofIP administration.
Histological examination has revealed extensive apoptosis of enterocytes in the apical villi of infants with NEC [23]. Activated TLR4 induces the activation of various transcription factors, including nuclear factor κ-light-chain-enhancer of activated B cells and interferon regulatory factor 3, which ultimately help eliminate invading microbes [24]. This facilitates the passage of infectious agents across the intestinal wall and triggers immune responses. Early TLR4 activation in the intestinal epithelium contributes to the depletion of enterocytes [25]. Additionally, it has been reported that intestinal TLR4 expression is upregulated in NEC [26], and in mice with an inactivating mutation in tlr4, NEC development is inhibited [26,27]. The abundance of IL-10-producing regulatory T cells decreases in the ileum of NEC mice, highlighting their anti-inflammatory properties [28]. Furthermore, IL-10 deficient mice exhibit severe epithelial damage and overall NEC injury [29]. The role of tumor necrosis factor (TNF)-α, an inflammatory cytokine, in various inflammatory conditions affecting the small intestine has been well-established [30]. Additionally, in NEC, there is an increase in the abundance of TNF-α in the intestinal tissues of affected patients [31].
Numerous attempts have been made to develop BM-MSC administration as a potential therapeutic strategy for NEC [18-20]. Studies have confirmed decreased histological injury scores and improved gut barrier function following IP or IV administration of BM-MSCs [19]. Moreover, administration of BM-MSCs increases the intestinal abundance of anti-inflammatory cytokines, including IL-10, while decreasing that of proinflammatory cytokines, such as TNF-α, in rats with induced NEC [20]. A recent meta-analysis has indicated that stem cells prevent NEC in experimental rodent models [32]. In the present study, consistent with the findings of previous studies performed using a mouse model [18-20], we confirmed that the administration of BM-MSCs resulted in a reduction in tissue injury, apoptosis, and inflammatory marker levels. The timing of BM-MSC administration was different in previous studies, ranging from before NEC induction to after its onset [18-20]. In our study, BM-MSCs were administered after NEC induction. This approach was employed to assess the effectiveness of BM-MSCs as a treatment agent even when administered after NEC onset, and the results showed that it was indeed effective.
To investigate whether the dose and frequency of BMMSC administration have an effect on the therapeutic efficacy, we conducted experiments by adjusting the amount and frequency of BM-MSCs administered IP and found that the therapeutic effects did not exhibit a dose-dependent relationship. Furthermore, even when BM-MSCs were administered at multiple divided doses, there was no improvement in outcomes. However, these results contradict the findings of a recent study in a mouse model, that is, BM-MSC administration reduces NEC severity in a dose-dependent manner [33]. Additional studies are required to determine the appropriate dose and frequency of BM-MSC administration for effective treatment of NEC.
We confirmed that the efficacy of oral administration of BM-MSCs is similar to that of IP administration in alleviating NEC. All experiments conducted thus far have involved the administration of BM-MSCs via the IP or IV route. Although previous studies have suggested that the IP route is the best way to introduce stem cells to treat gastrointestinal disorders [34], IP injections are considered invasive in premature infants. Additionally, maintaining an IV line for stem cell injection poses potential risks such as occlusion, thrombosis, breakage, migration, displacement, and infection, which can be fatal to premature infants [35]. The oral administration of stem cells has major clinical advantages over IV or IP routes in vulnerable patients.
Our study was limited by the absence of a comparison of BM-MSC administration orally at varying doses and frequencies. This was because our primary focus was on assessing the effectiveness of oral BM-MSC administration for NEC treatment. Consequently, further studies are required to compare and analyze the oral administration of BM-MSC at different dose and frequencies.
In conclusion, the oral administration of BM-MSCs holds promise as a potential treatment strategy for neonates with NEC. Further human studies with BM-MSCs are required to establish the optimal dose to ensure both safety and treatment effectiveness.
Supplementary materials
Supplementary Fig. 1 can be found via https://doi.org/10.3345/cep.2023.01151.
Experimental design of in vitro wound healing assay. The groups were separated as follows: group 1 (control), group 2 (NEC+PBS), and group 3 (NEC+BM-MSCs). Photomicrographs were taken at 0, 4, 10, and 24 hours after the creation of the wound. NEC, necrotizing enterocolitis; PBS, phosphate-buffered saline; BM-MSC, bone marrow-derived mesenchymal stem cell; LPS, lipopolysaccharide.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: LJ, JYH; Formal Analysis: LYS, LJ; Investigation: LYS, LJ; Methodology: LJ; Project Administration: LJ, Yong Hoon Jun; Writing–Original Draft: LYS, LJ; Writing–Review & Editing: LYS, Yong Hoon Jun, LJ
Acknowledgements
We thank SCM Life Science Inc. for assistance in preparing BM-MSCs used in this study.

