Iron deficiency in children with a focus on inflammatory conditions
Article information
Abstract
Iron deficiency (ID) tends to be overlooked compared with anemia. However, its prevalence is estimated to be twice as high as that of ID anemia, and ID without anemia can be accompanied by clinical and functional impairments. The symptoms of ID are nonspecific, such as fatigue and lethargy, but can lead to neurodevelopmental disorders in children, restless legs syndrome, and recurrent infections due to immune system dysregulation. In particular, the risk of ID is high in the context of chronic inflammatory diseases (CIDs) due to the reaction of various cytokines and the resulting increase in hepcidin levels; ID further exacerbates these diseases and increases mortality. Therefore, the diagnosis of ID should not be overlooked through ID screening especially in high-risk groups. Ferritin and transferrin saturation levels are the primary laboratory parameters used to diagnose ID. However, as ferritin levels respond to inflammation, the diagnostic criteria differ among guidelines. Therefore, new tools and criteria for accurately diagnosing ID should be developed. Treatment can be initiated only with an accurate diagnosis. Oral iron is typically the first-line treatment for ID; however, the efficacy and safety of intravenous iron have recently been recognized. Symptoms improve quickly after treatment, and the prognosis of accompanying diseases can also be improved. This review highlights the need to improve global awareness of ID diagnosis and treatment, even in the absence of anemia, to improve the quality of life of affected children, especially those with CIDs.
Key message
· Iron deficiency has important effects on neurodevelopment and the immune system in children.
· Hepcidine plays an important role in iron homeostasis.
· Diagnosis and treatment of iron deficiency in chronic inflammatory disease are important for patients' quality of life and disease course.
Introduction
Iron deficiency (ID) and iron deficiency anemia (IDA) are health-related conditions and among the leading contributors to the global burden of disease. Worldwide, IDA affects an estimated 1.2 billion people. Although the statistics for ID are unclear, its prevalence is considered at least double that ofIDA [1,2].
Iron is an essential element in numerous physiological processes and the functioning of all organs [3]. Since most consumed is used for hemoglobin synthesis, IDA can be said to be the ultimate outcome of ID; however, in the same vein, clinical and functional impairments can occur even without anemia. Therefore, ID can be viewed as a broader condition than IDA. Nevertheless, ID without anemia is often overlooked [1,4].
ID is associated with risks in children, including fatigue, impaired cognitive and brain development, failure to thrive, restless legs syndrome (RLS), immune dysregulation, and cardiac problems [5]. In particular, ID is a common comorbidity in patients with chronic inflammatory diseases (CID), such as congestive heart failure, inflammatory bowel disease (IBD), and chronic kidney disease (CKD) [6]. ID reportedly adversely affects the aggravation of concomitant diseases and prognosis [6]. However, there are many cases in which ID is masked by other diseases.
ID is an important global disease in an era that prioritizes children’s quality of life. Therefore, this review aimed to provide an overview of the current knowledge of the diagnosis and causes of ID with a focus on the problems associated with ID in the context of chronic inflammation.
ID etiology and classification
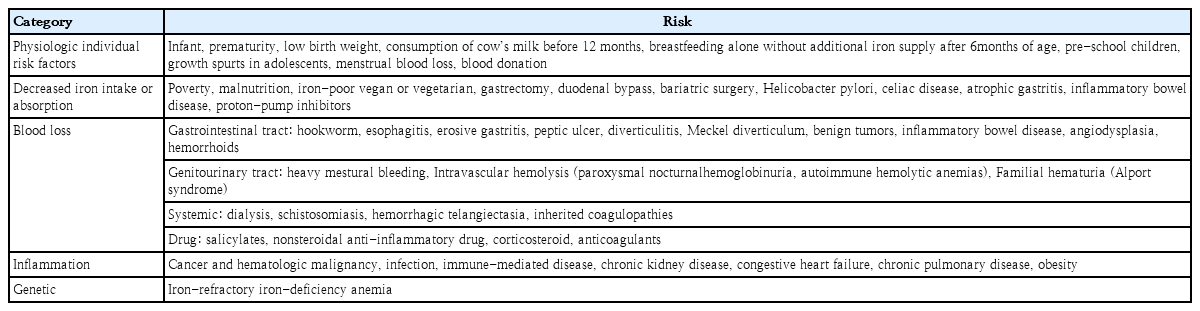
Alterations of iron availability can be classified in terms of “absolute” or “functional” ID. In the absolute ID state, the total body iron stores, mostly in macrophages and hepatocytes, are reduced. Absolute ID can occur in cases of increased demand, decreased iron intake or absorption, or chronic blood loss (Table 1). Absolute ID is characterized by low levels of stored iron, resulting in low serum ferritin and reduced transferrin levels [7].
Unlike absolute ID, functional ID is a state of imbalance between iron demand and serum iron availability that may occur despite adequate body iron stores [8]. This phenomenon is caused by an insufficient release of iron due to the internalization of ferroportin (FPN) channels by hepcidin [7]. Functional ID is most frequently observed in the systemic inflammation and/or infection setting (Table 1). Because iron-deficient erythropoiesis occurs by reducing iron bioavailability, the term functional ID is sometimes used interchangeably with iron-restricted erythropoiesis.
Some patients with CID have both functional and absolute ID. However, an accurate diagnosis is important because the treatment plans differ accordingly.
Metabolic pathology of ID, especially in inflammation
Iron balance is important for all cells, and iron homeostasis is precisely regulated. Iron availability is tightly regulated at the cellular and systemic levels through coordination with the expression of iron importer and exporter, and iron storage. ID is an inevitable side effect that occurs when iron availability is insufficient [1,4]. When ID occurs due to multiple causes (Table 1), adaptive mechanisms optimize iron use and aid its absorption.
1. Iron adaptation at a systemic level
The mechanism of systemic iron adaptation is regulated by hepcidin-based homeostatic control [1]. Hepcidin is a small peptide hormone primarily synthesized in the liver. This protein binds to the iron exporter FPN and blocks iron efflux into the plasma. FPN is highly expressed in enterocytes and macrophages, and hepcidin induces the degradation of iron-containing FPN, blocking the iron efflux pathway [1].
When ID occurs, hepcidin is suppressed by iron supply optimization via iron absorption enhancement and recycling. Several mechanisms downregulate hepcidin transcription in hepatocytes (Fig. 1A). First, the bone morphogenetic protein (BMP)-SMAD signaling pathway is repressed by low levels of BMP6. Transmembrane serine protease 6 disrupts hepcidin synthesis by cleaving hemojuvelin, a coreceptor of BMP [9]. In addition, the ALK3 signal is reduced because transferrin receptor 2 is not stabilized on the cell surface, which in turn reduces hepcidin transcription. Histone deacetylase 3 participates in hepcidin suppression by erasing the markers of hepcidin activation [10]. Additionally, increased erythroferrone blocks hepcidin transcription via an unknown mechanism [3]. The reduction in hepcidin levels by these mechanisms increases FPN activity and consequently increases the amount of iron entering the plasma [11].
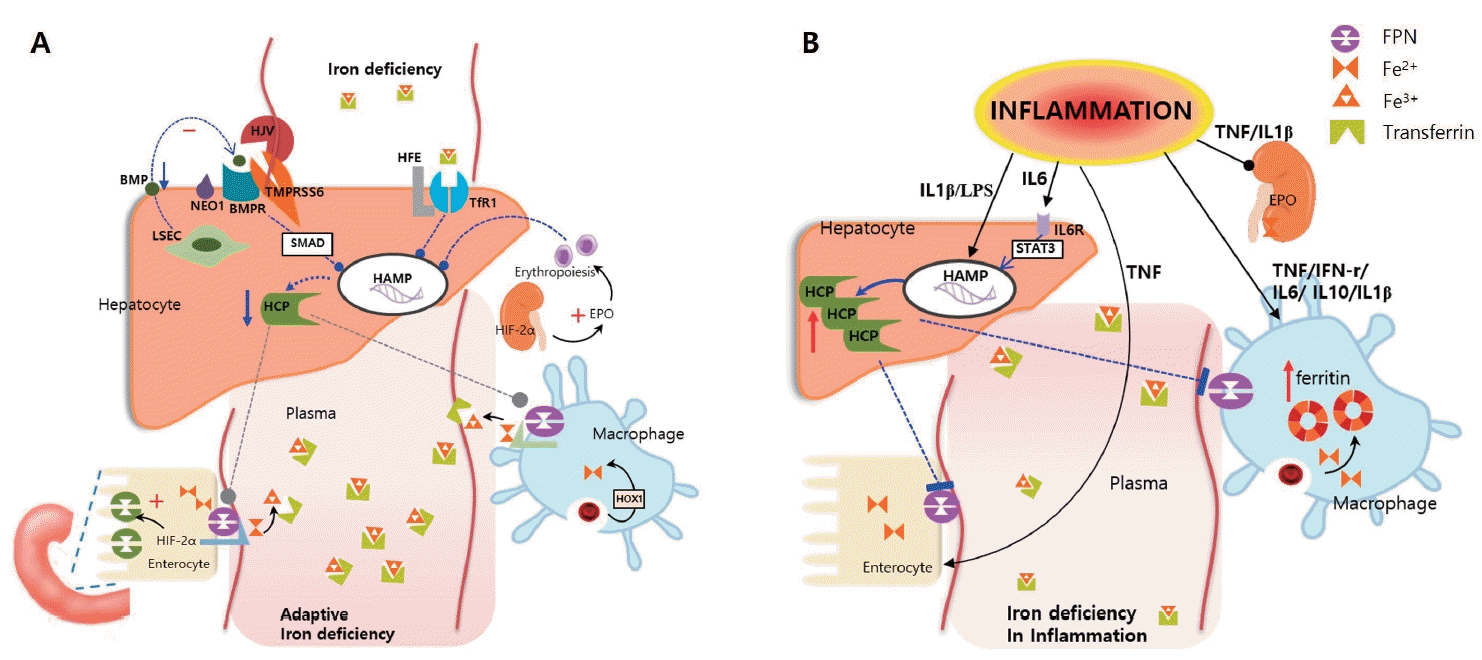
Hepcidin, produced in the liver, plays a key role in iron regulation. Hepcidin blocks the export of iron from the cell to the plasma by degrading ferroportin (FPN) in enterocytes, macrophages, and hepatocytes. (A) Adaptation to iron deficiency: Several mechanisms interfere with hepcidin production. The bone morphogenetic protein (BMP)-SMAD signaling cascade and ALK3 (activin receptor-like kinase 3) signaling are repressed. Increased erythroferrone blocks the hepcidin pathway via an unknown mechanism. In addition, iron deficiencyinduced hypoxia-inducible factor-2α (HIF-2α) increases divalent metal transporter 1 expression to increase the transfer of dietary iron and the production of erythropoietin (EPO) to stimulate erythropoiesis. (B) In the inflammatory state, several cytokines (interleukin [IL]-6, IL- 1β, lipopolysaccharide) increase hepcidin production. In addition, cytokines such as tumor necrosis factor (TNF)-α and interferon (IFN)-γ stimulate the production of ferritin in macrophages, allowing iron to remain in the cells and eventually reducing plasma iron levels. LSEC, liver sinusoidal endothelial cells; NEO1, neogenin; BMPR, bone morphogenic protein receptor; TMPRSS6; transmembrane serine protease 6; HFE, hephaestin; HOX1, heme oxygenase 1; HCP, hepcidin; HAMP, hepcidin gene; STAT3, signal transducer and activator of transcription-3; TfR1, transferrin receptor 1; LPS, lipopolysaccharide.
Additionally, erythropoietin (EPO) production stimulates erythropoiesis in erythroblasts. In enterocytes, ID-induced hypoxia-inducible factor-2α (HIF-2α) upregulates the expression of divalent metal transporter 1 (DMT1) to increase dietary iron uptake [3].
2. Iron adaptation at a cellular level
Intracellular iron homeostasis is regulated by iron regulatory proteins (IRPs) that bind to the iron-responsive element (IRE) [11]. IRE resides in the untranslated region (UTR) of messenger RNA (mRNA) and synthesizes the proteins involved in iron metabolism. When iron is insufficient in cells, an IRP binds to the IRE present in the 5′-UTR and interferes with mRNA transcription, reducing the synthesis of ferritin, FPN, and HIF-2α while binding to the IRE present in the 3′-UTR increases the synthesis of transferrin receptor 1 (TfR1) and DMT1 [11]. In addition, inhibition of the mammalian target of rapamycin activates tristetraproline, which reduces both TfR1 and FPN expression and conserves the iron required for tissue metabolism [4].
In ID, cells can recover the iron stored in ferritin by degradation (ferritinophagy) via nuclear receptor coactivator 4 [3]. Ferritin plays an important role in intracellular iron storage.
3. Iron adaptation in inflammatory condition
Several inflammatory cytokines are released by cells in the immune system that respond to microbial molecules, autoantigens, or tumor antigens, which alters whole-body iron metabolism [12]. First, proinflammatory cytokines affect hepcidin production and release. Interleukin (IL)-6, IL-1β, and lipopolysaccharide are potent inducers of hepcidin; however, they reduce the expression of the iron transport protein transferrin [12]. Among them, IL-6 is considered the most important factor that stimulates hepcidin production through STAT3 [13]. Consequently, FPN is degraded, iron is retained in macrophages, and the efflux of absorbed iron from the duodenum is blocked. In addition, several cytokines (IL-1β, IL-6, IL-10, and interferon-gamma [IFN- γ]) promote iron uptake into macrophages via transferrin receptor–mediated endocytosis [14]. Because this can increase radical-mediated damage, they stimulate ferritin production to ensure safe and efficient iron storage. In addition, these cytokines reduce FPN expression by inhibiting its transcription [15]. Another cytokine, tumor necrosis factor-α (TNF-α), decreases duodenal iron absorption through a hepcidin-independent mechanism that has not yet been precisely elucidated [12]. These events induce iron restriction at the cellular level by the storage of iron as ferritin and prevention of its outflow into the plasma, thereby causing ID (Fig. 1B).
Diagnosis of ID
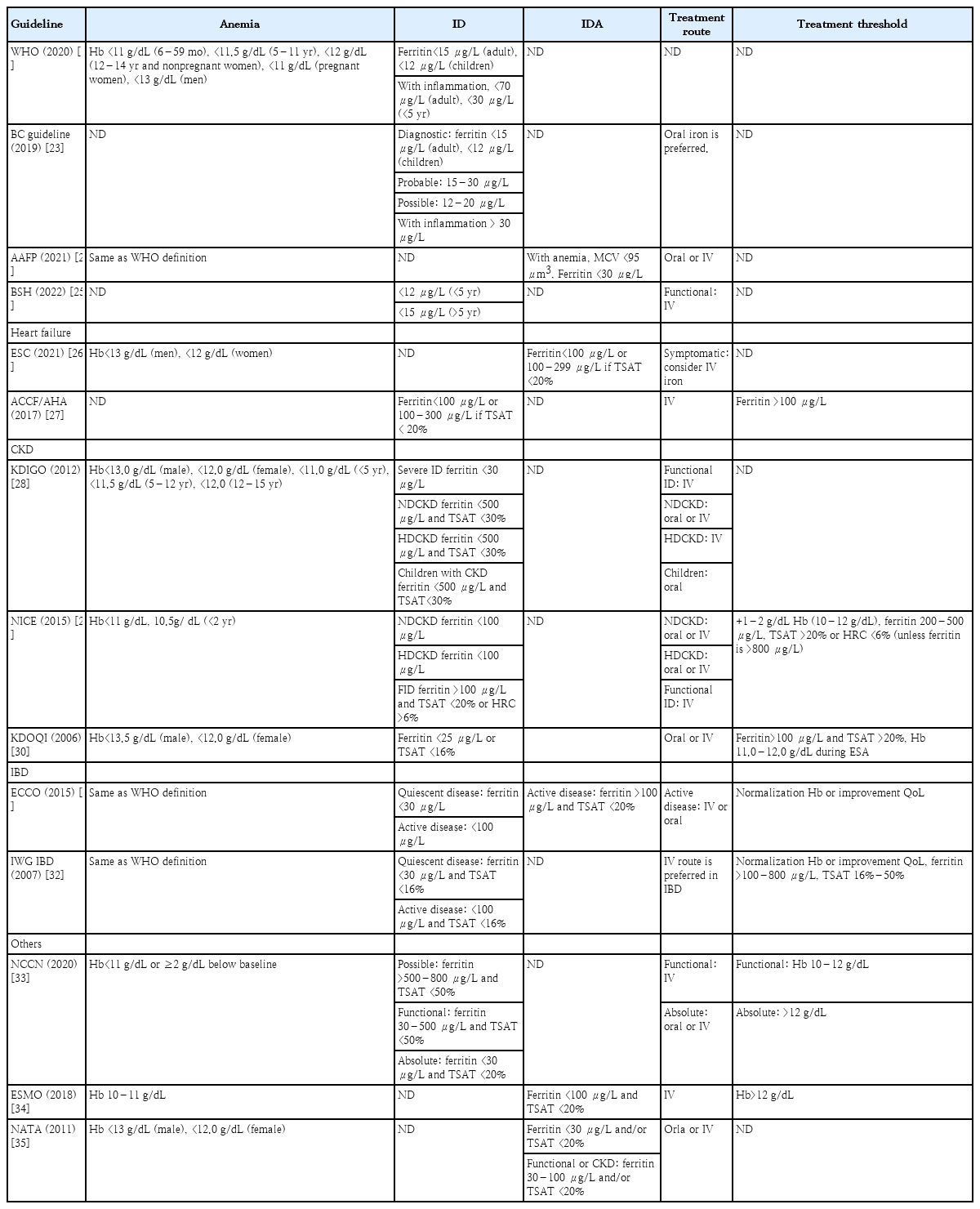
In the diagnosis of ID, the bone marrow is a suitable tissue for evaluating iron deposits. However, bone marrow aspiration or biopsy is both invasive and expensive [16,17]. Bone marrow-based detection has been replaced by noninvasive blood biomarkers such as ferritin, serum iron, and transferrin saturation (TSAT) [16]. Among these, serum ferritin is the most sensitive and widely used diagnostic marker for ID [18]. Ferritin is a protein that stores iron within cells and is found in all cell types. Ferritin can store up to 4,500 iron atoms; when the intracellular iron concentration increases, ferritin exerts a powerful ability to bind to iron, preventing the cellular damage caused by free iron [19]. A small amount of ferritin is released from the cell and is present in the plasma. This concentration is closely related to the intracellular iron concentration; therefore, blood ferritin concentration is an indicator of the amount of iron stored in the body [16]. Normal ferritin concentrations vary with age and sex, even in the absence of inflammation or liver disease [16]. A ferritin level <12–15 μg/L confirms a diagnosis of ID, although a ferritin level <30 μg/L has been more widely used recently to identify ID with a sensitivity of 92% and specificity of 98% [16,20]. However, ferritin is an acute-phase reactant that increases in response to cellular damage and inflammation as highlighted by reports of extremely high serum ferritin concentrations associated with severe cases of coronavirus disease 2019 [21]. Even if the ferritin concentration is high,ID cannot be completely ruled out. This may reflect a complex interaction between ID and inflammation or other causes of cell damage [22]. Therefore, ferritin alone is rarely used to confirm the ID diagnosis in patients with comorbid inflammatory components. Several studies and guidelines have offered different opinions on the most appropriate ferritin threshold (Table 2) [16,23-35].
Serum iron concentration is decreased in both ID and inflammatory conditions; therefore, it alone cannot be an indicator of ID [1]. TSAT is useful for defining plasma iron availability. Many guidelines recommend TSAT as a diagnostic or therapeutic tool when ferritin levels are unreliable, with a level of <16% in normal cases and <20% in cases with inflammatory conditions serving as the diagnostic criteria for ID (Table 2). Traditionally, the diagnostic cutoff values for ID in patients with CID are TSAT <20% and serum ferritin <100 μg/L as recommended in many guidelines. However, several studies have shown that these criteria are insufficiently sensitive to detect ID in patients with CID [36]. In a study of 100 patients with CKD (stages 3–5), these indices identified only 17% of patients with ID, whereas approximately 50% of these patients were deemed iron-deficient based on the gold-standard diagnostic method of bone marrow iron staining [36]. Therefore, it is necessary to consider alternative diagnostic methods to confirm iron storage depletion in patients with CID.
Among the diagnostic methods that are not widely used but are used as complementary tests, soluble transferrin receptor levels, and the log ferritin ratio are useful biomarkers for predicting bone mass iron stores [37]. Reticulocyte hemoglobin content and hypochromic red blood cell percentage are useful diagnostic tools for detecting ID [37]. In addition, serum hepcidin levels are decreased or undetectable in ID but markedly increased in ID during inflammation, providing a useful test for differentiation [4].
Clinical importance of ID in children
Representative manifestations of ID include constitutional symptoms such as fatigue, lethargy, dizziness, and poor concentration, although ID can also be asymptomatic. The main symptoms and complications are summarized in Table 3. In addition, clinical and functional impairments can occur even in the absence of anemia. Therefore, the screening of target groups and appropriate treatments are needed.
1. Neurodevelopmental deficits
Iron plays an important role in brain and cognitive development [2]. Brain development accelerates in the 3rd trimester of pregnancy, for which sufficient iron is required. ID during this period can result in neurological and cognitive deficits, delayed motor development, deficits in auditory memory and learning, and emotional disorders [38]. Although it remains unclear precisely which areas of the brain are affected by ID, several studies have suggested that ID impairs energy metabolism and affects hippocampal neuron development [38]. Other neurodevelopmental processes known to be disrupted by ID include myelination, dendriogenesis, synaptosis, and neurotransmission [39].
In children, ID leads to delayed cognitive, motor, attention, and memory deficits; visual and auditory deficits; decreased school performance; and/or behavioral disorders, some with persistent long-term effects. In adolescents, ID can lead to decreased psychomotor skills and concentration, thereby affecting school performance. The relationship between attention-deficit/hyperactivity disorder (ADHD) and ID has been suggested in several studies [40]. First, the ferritin levels of children with ADHD were reportedly lower than those of children without ADHD, and ADHD symptoms improved with iron supplementation [40]. Second, it been hypothesized that ID may cause dysregulation of central dopaminergic neurotransmission, a pathological cause of ADHD [41].
In addition, breath-holding spells increase in ID, and the frequency of which decreases with iron therapy [42]. RLS is a relatively common neurological disorder associated with ID. An association between iron metabolism and dopaminergic neurotransmission disorders in the striatum, the presumed cause of RLS, has been suggested in animal models [43]. In addition, 2 genes (MEIS1 and BTBD9) were identified in a genomic analysis related to RLS that appears to affect both the expression of periodic limb movements during sleep and iron homeostasis [44]. For this reason, the International Restless Legs Syndrome Study Group reported iron supplementation when ferritin levels are <50 μg/L [45].
2. Dysregulation of immune and respiratory systems
Iron is essential for the immunosurveillance systems as well as tissue proliferation, such as the intestinal epithelium, which serves as a physical barrier to infection [46]. In addition, iron status directly impacts innate immunity and indirectly impacts adaptive immunity [47,48]. Therefore, iron homeostasis is important for the normal development of the immune system and immune cell differentiation.
Macrophages are the primary cells for iron transmission, distribution, and mobilization; therefore, ID status directly impacts innate immunity [47,49]. ID reduces the distribution capacity of macrophages, leading to a transition toward a proinflammatory phenotype and low-level inflammation with decreased production of macrophage inhibitory factors [47,49]. ID also causes a shift in monocytes and decreases monocyte phagocyte activity in patients with IDA [48]. The development, proliferation, activation, and function of neutrophils and natural killer cells are also reportedly affected by ID [48]. For example, myeloperoxidase activity is reduced, and intracellular bactericidal activity may be impaired in patients with ID [49-51].
Second, ID interferes with adaptive immunity to a relatively low degree. ID reduces the counts of T lymphocytes, affects their differentiation, and reduces CD4+ and CD8+ T-cell numbers in the circulation, or it alters the proliferation of T helper (Th)-1 and Th-2 subsets. T-cell activation is controlled by TFR1 (CD71)-mediated iron uptake via an IL-2-dependent pathway. Therefore, TFR1 mutations can lead to defects in iron endocytosis and T-cell dysfunction, which can result in combined immunodeficiency diseases [48,52]. The lack of iron contributes to downregulation of the antibody response. Recent studies reported that serum iron levels affect antibody formation following vaccination [53]. In addition, although controversial, another study demonstrated that the concentrations of immunoglobulin G subgroups are reduced in patients with ID [54].
Other studies suggested that these immunological impairments are due to the dysregulation of IL and other cytokines (e.g., IL-6, IL-2, TNF-α, IFN-γ, and IL-10) in patients with ID [49-51]. In particular, serum IL-6 level was significantly lower in patients with ID, and T-cell dysfunction may be the result of low cytokine activity [55]. In addition, impaired IL-2 production by lymphocytes was observed in children with ID [56].
ID is associated with several immune diseases and affects host susceptibility to infection.In particular,ID is a risk factor for the development of recurrent respiratory tract infections and has been associated with several lung diseases, such as chronic obstructive pulmonary disease, lung cancer, cystic fibrosis, idiopathic pulmonary fibrosis, and asthma [57]. Several studies have shown that lower respiratory tract infections occur more frequently in the presence of ID, a risk that is significantly reduced by iron supplementation [57,58]. Furthermore, the observed risk could be due to the low oxygen-carrying capacity in the pulmonary vasculature and parenchyma that leads to a low level of protection against invading pathogens [58]. Other studies reported an association between low serum iron levels and abnormal lung function with increased high and small airway resistance [59]. Moreover, several studies reported that urinary tract infections, intensive care unit–acquired infections, postoperative infections, and otitis media are more common in patients with ID [46].
3. Impact on CID
Multifactorial ID causes are common comorbidities in patients with CID. ID is a comorbidity that exacerbates the underlying disease and increases mortality rates.
One study identified ID in 56% of pediatric patients with heart failure, and ID was associated with an increased risk of ventricular assist device implantation, heart transplantation, and death.In addition,this study suggested that ID resulted in a significantly shorter time of onset for these composite adverse events [60]. Another pediatric cohort study reported that low serum iron levels were associated with inpatient hospitalization, inotropic medication use, or worse heart failure severity, while low ferritin levels were associated with increased mortality rates [61]. These studies suggest that ID may be an actionable biomarker and a potential new therapeutic target in the field of pediatric heart failure. In addition, the use of anemia alone as the threshold for treating ID may be insufficient, thereby prolonging compromised tissue function and delaying treatment initiation [60,61]. Other studies argued that ID is a poor prognostic factor for heart failure, even in the absence of anemia [62]. Therefore, routine and repeated screening for ID at the time of diagnosis and every 3–6 months thereafter should be performed in pediatric patients with heart failure [60].
ID is a major complication in pediatric patients with CKD. The prevalence ofID in patients with CKD is as high as 85% and increases as CKD progresses [6]. In a Korean cohort study of children with CKD, the disease history, systolic blood pressure, and presence of comorbidities were significantly higher in patients with versus without ID [63]. In this cohort study, ferritin levels increased with increasing CKD stage, whereas transferrin levels did not change. This suggests that ferritin levels can be increased by both inflammation and ID in pediatric patients with CKD [63]. Unlike heart failure, many CKD-related guidelines focus more on anemia than on ID [28,29]. However, ID plays an essential role in anemia in patients with CKD. This is due to both a lack of iron storage (absolute ID) and relative (functional) ID: The chronic inflammatory state in CKD reduces erythropoiesis in the bone marrow, reduces EPO production in the kidney, and increases hepcidin production in the liver, thereby inhibiting iron absorption from the gut and its release from iron-storing cells [64,65]. Increased levels of proinflammatory cytokines such as IL-6 and TNF-α affect this mechanism [64].
The prevalence of ID is reportedly as high as 80%–90% in patients with IBD [18]. The mechanism involves chronic blood loss in the gastrointestinal tract, impaired iron absorption in the intestine, and chronic proinflammatory cytokines that block iron transport and increase hepcidin production [6]. Although ID can be more easily corrected before anemia occurs, IBD treatment also focuses on evidence of anemia [66]. Additionally, patients with IBD are generally not treated appropriately for ID during disease activation, and the ID status usually does not recover even if remission is achieved. However, even in patients with IBD, iron replacement therapy for ID without anemia improves the quality of life [67].
Current issues in the treatment of ID
The treatment for IDA, as well as ID, is iron supplementation. Treatment should always be initiated when a precise laboratory diagnosis is established. Appropriate nutritional recommendations and corrections for ID-causing diseases are also necessary.
1. ID without complex condition
There is evidence that all patients diagnosed with ID, especially those who are symptomatic, should be adequately treated with iron, even in the absence of anemia [17]. Clinically, iron intake in patients with non-anemic ID reduces fatigue self-scores [68]. Iron also improves maximal exercise performance and aerobic capacity [69]. Another review showed that iron supplementation reduced RLS/periodic limb movement disorders [70]. Iron supplementation improved cognitive performance in children aged 5–12 years and improve verbal learning and memory in adolescent girls aged 13–18 years [71,72].
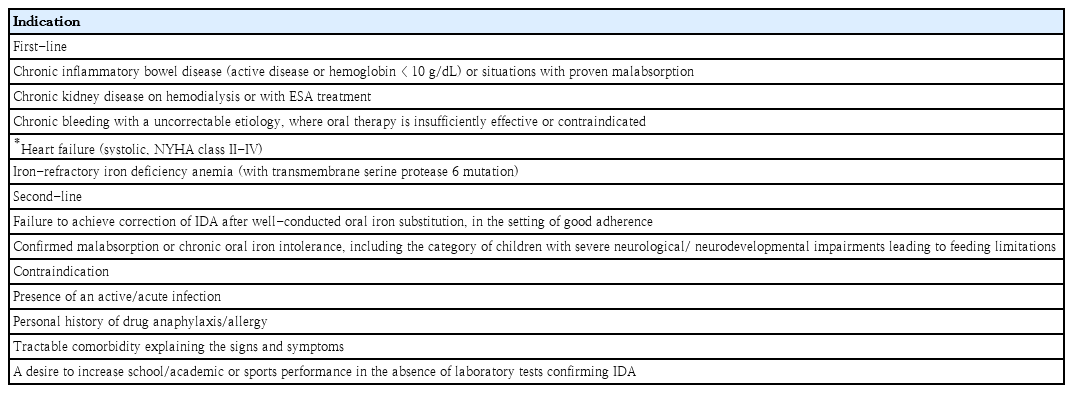
Most guidelines recommend oral iron therapy as first-line treatment, even in children, owing to its effectiveness, ease of administration, and low cost. Ferrous sulfate, fumarate, gluconate, ascorbate, lactate, succinate, and glycine sulfate are typically used as oral iron supplements. One or 2 doses of iron at a total daily dose of 3–6 mg/kg are adequate; the maximum dose is 150–200 mg of elemental iron [9]. Ideally, oral iron should be administered for at least 3 months to completely replenish iron stores, defined as reaching a serum ferritin level ≥50 ng/mL. However, common side effects such as epigastric discomfort, nausea, diarrhea, and constipation, which are not severe, are reported in 30%–70% of cases of iron supplementation [9]. For these reasons, drug compliance is poor or treatment fails, and iron supplementation can be administered intravenously. The indications for parenteral iron therapy are shown in Table 4.
2. ID with complex condition(s)
Parenteral iron is recommended as the primary treatment for many cases ofID with complex medical conditions. Oral iron therapy is ineffective in chronic diseases because it increases hepcidin synthesis owing to inflammation and blocks the absorption of oral iron by enterocytes [6].
For patients with heart failure, effort is required to reduce the readmission rate, which is directly related to mortality. Several studies have demonstrated that reducing the incidence of ID can reduce hospitalization and treatment costs for patients with heart failure [73]. In addition, the most common comorbidity in heart failure is ID, which is relatively easy to diagnose, and its treatment is simple and effective [73]. Therefore, the early identification and treatment of ID have been suggested in many heart failure guidelines [26,74]. The 2021 European Society of Cardiology guidelines recommended that intravenous iron therapy be considered in patients with chronic heart failure (left ventricular ejection fraction ≤45%) and ID (defined as ferritin<100 μg/L or ferritin 100–300 μg/L with TSAT<20%) [26]. The American College of Cardiology and American Heart Association guidelines also recommend intravenous iron therapy for adult iron-deficient patients with heart failure [27]. In pediatric patients with heart failure, the role of intravenous iron therapy was emphasized in a recent study that revealed the poor efficacy of oral iron replacement therapy [74].
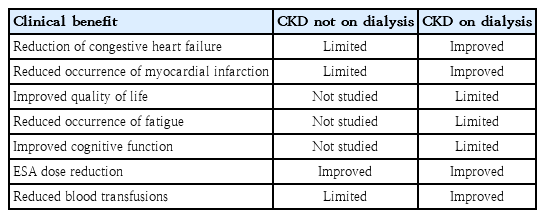
Almost all patients with CKD suffer from absolute and functional ID via various mechanisms; consequently, parenteral iron produces higher results and requires a greater reduction of the required doses of EPO agents than oral iron in patients with CKD [64,65]. TSAT and ferritin levels suggest that the start or end of treatment varies among guidelines (Table 2). In CKD, absolute ID is defined as a TSAT <20% and ferritin <100 mg/L in patients not on hemodialysis (NDCKD) or <200 mg/L in hemodialysis (HDCKD) patients. Functional ID was defined as a TSAT <20% and a ferritin >100 mg/L in patients with NDCKD or >200 mg/L in patients with HDCKD [75]. The Proactive IV Iron Therapy in Hemodialysis Patients study was a randomized controlled trial of more than 2,000 patients in which the administration of high-dose (400 mg)intravenous iron to dialysis-dependent CKD patients with ID (serum ferritin <700 μg/L and TSAT ≤40%) reduced all-cause death, nonfatal myocardial infarction, nonfatal stroke, and heart failure hospitalization rates [76]. The clinical benefits of iron supplementation in CKD patients are presented in Table 5. However, although many studies have shown the safety of intravenous iron, there are limitations regarding whether it is safer than oral iron supplementation, especially in patients without dialysis [65].
Although the correction of iron status in IBD patients is known to significantly improve quality of life, >50% of ID patients, even those with anemia, do not receive adequate iron treatment [67]. A systematic review found that, in patients with IBD, parenteral iron improved the hemoglobin concentration more than oral iron with no serious adverse events [77]. Since oral ferrous supplements are oxidized in the gut, they form activated hydroxyl radicals and affect the mucosa, exacerbating gastrointestinal symptoms such as nausea and pain in patients with IBD [78]. A recent study conducted in Switzerland also reported that intravenous iron featured less withdrawal due to adverse events than oral iron, demonstrating a good effect; among the administered irons, ferric carboxymaltose was the most cost-effective [79]. A similar finding was reported in children with IBD; both ID and IDA resolved after intravenous iron infusion without serious side effects, and no C-reactive protein elevation was observed [78].
In the 2018 European Society for Medical Oncology guidelines, absolute ID was defined as a serum ferritin level <100 μg/L, while functional ID was defined as a serum ferritin level ≥100 μg/L and TSAT level <20% in patients with cancer [34]. According to the above definition, in the recently reported CARENFER study, 86%–90% of cancer patients were diagnosed with ID [80]. In addition, this guideline recommends ID treatment as soon as possible when the ferritin level is <100 μg/L and the TSAT level is <20% regardless of anemia status [34]. In the National Comprehensive Cancer Network guidelines, oral or intravenous iron administration for absolute ID is always recommended, and functional ID is considered in cases of treatment failure. For functional ID, intravenous iron administration should be considered [33].
As for adults, the International Restless Leg Syndrome Study Group task force recommends that intravenous iron be considered for RLS or periodic limb movement disorder in children [45]. In a pediatric patient analysis report, intravenous iron supplementation improved the clinical severity and laboratory parameters of RLS and periodic limb movement, and the treatment was well-tolerated [81]. The target of iron therapy in pediatric sleep disorders is a serum ferritin level ≥50 μg/L [45].
Conclusion
Over the past few decades, global awareness has increased of anemia and its consequences on children’s health and development. Accordingly, it is now time to increase interest in and awareness of ID. Global awareness should be changed to consider ID a disease itself rather than simply a category of malnutrition or cause of anemia. ID is a common and treatable disease that is often overlooked. Therefore, along with improving its recognition of ID, presenting improved guidelines for its diagnosis and treatment will be a shortcut to improving the quality of life of many children worldwide, especially those with chronic diseases.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.